

Confocal technology is one of the most important advances in optical microscopy, and many disciplines within Texas A&M AgriLife and other parts of The Texas A&M University System are discovering it can also be a game-changer in their research.
Brian Shaw, Ph.D., a professor in the Department of Plant Pathology and Microbiology, PLPM, at the Texas A&M College of Agriculture and Life Sciences is an expert in confocal microscopy. He not only applies this state-of-the-art technology in his own research, but also encourages undergraduate and graduate students, as well as other researchers, to use it to advance their own scientific efforts.
What is confocal microscopy?
“Confocal microscopy offers several advantages over conventional microscopy, including the ability to control depth of field and to reduce or eliminate out-of-focus light in image formation on the focal plane,” Shaw explains.
“It also gives you the ability to work with living cells and see the changes they make in real time, as well as to collect serial sections from thick specimens,” he said. “Additionally, it can produce an almost 3D image of the cell, allowing you to view it from various perspectives.”
Shaw’s primary confocal microscope, which allows for a wide range of imaging modalities, is located in the Plant Pathology and Microbiology Building on the Texas A&M campus.
It is an Olympus FV3000 confocal laser-scanning microscope with four detectors and six laser lines, which allow for simultaneous imaging of four-color channels of almost any fluorophore. It is fully motorized with autofocus, allowing for time-lapsed imaging for hours without losing focus. The microscope’s precision motorized stage makes it possible for multiple-point time-lapse of various specimens simultaneously.
Confocal microscopy and determining pathogenic infection
According to research in the Multidisciplinary Journal of Microbial Ecology, the official Journal of the International Society for Microbial Ecology, more than 80% of crop diseases are caused by fungi or fungus-like pathogens. These diseases lead to billions of dollars in crop losses and threaten food security.
Shaw said much of the research he and his team do relates to determining the cellular machinery involved in the growth and development of fungi and how fungal pathogens work.
“We are investigating surface characteristics of fungal spores and how they influence spore dispersal,” he said. “The fungal spore is the dormant cell that these organisms use to disperse across distance and through time.”
Shaw said fungi require polarized hyphal growth to have an impact on plants and humans, so understanding how hyphae are made is a fundamental concern. A hypha is the basic unit of a filamentous fungus and typically consists of a chain of elongated cells that expand at the apex of the tip cell. The elongated, thread-like cells grow only at their highly polarized apex and their growth is characterized by the initial establishment of one growth site, which is followed by its continuous maintenance.
“Our lab examines the temporal and spatial dynamics of cytoskeletal components during the growth and development of fungal hyphae,” he said. “Using the confocal microscope, we found that the spores of the important corn pathogen, Colletotrichum graminicola, are asymmetric. And these spores can only attach to their new host on one face of the spore.”
Shaw said since attachment to their new host is necessary to begin the disease cycle in corn, this discovery identifies a new and essential target to disrupt fungal disease in corn.
“With the help of this higher-resolution, almost 3D imagery, we were able to infer a correlation between the site of attachment and the site of infection by the fungal pathogen,” he said. “Confocal microscopy allows us to see these structures and their components at a level of detail not previously available. Now we can get a better picture of how fungi grow and identify the hyphae of specific fungi and their role in disease initiation.”
Undergraduate and graduate student use of confocal microscopy
Oli Bedsole, a graduate student in the Department of Plant Pathology and Microbiology who works in Shaw’s laboratory, said he uses confocal microscopy to study how different proteins interact and associate in fungi.
“I am studying the adhesive in fungal spores that allows them to attach and later infect plants, like corn,” Bedsole said. “The resolution from a confocal is paramount and allows me to see where the adhesive is located, where specific proteins are and how they interact.”
Bedsole said he learned the basics of confocal microscopy from Shaw and other lab members, then received further training through the graduate course Theory and Applications of Light Microscopy.
“We almost exclusively image live cell samples and get incredible resolution,” he said. “Confocal microscopy allows us to make 3D constructions, giving us a unique perspective and clarity. It is also a powerful tool for helping us communicate our science to others since they can see this 3D image.”
Mary Cowser, an undergraduate working with Bedsole, said the imagery from confocal microscopy helps bring the science to life.
“Creating images with vibrant colors by staining different parts of fungal structures gives us an image of that structure enhanced with color,” she said. “And being able to see structures in 3D as they grow in real time is fascinating and can give a lot of insight into how they operate. Having these images helps us learn more about pathogens and their mechanisms, which benefits the environmental sciences.”
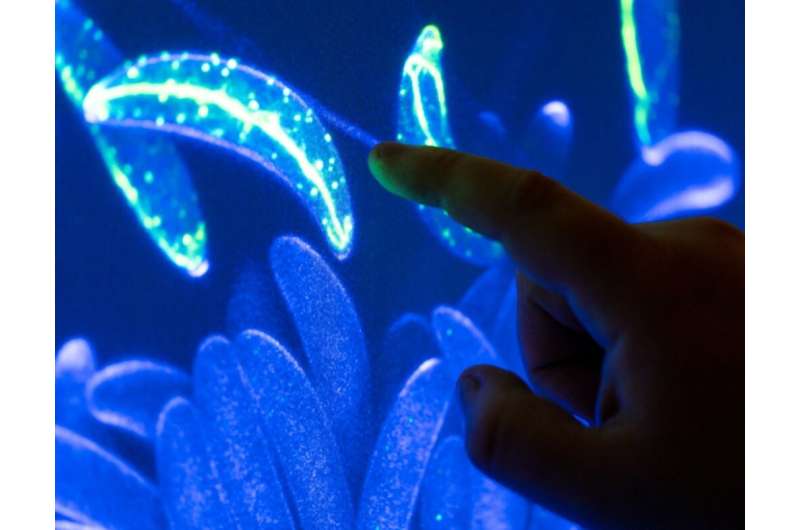
Bedsole, Cowser and others in Shaw’s classes receive specialized training and diverse experiences relating to biological science techniques.
“There are a variety of applications for confocal microscopy in microbiology and the environmental sciences,” Shaw said. “Having training on the use of this technology provides an extra level of experience and expertise for environmental sciences majors, and the technology itself can be of great benefit to them should they pursue a career in which they address biological issues in the environment.”
Multidisciplinary use of confocal microscopy at Texas A&M
Shaw said in addition to applications within plant pathology, microbiology and the bioenvironmental sciences as a whole, confocal microscopy benefits other academic disciplines.
Shaw and his team have collaborated with other Texas A&M researchers on the interdisciplinary and multidisciplinary use of this technology to advance science.
For example, Matthew Sachs, Ph.D., a professor in the College of Arts and Sciences Department of Biology, and graduate student Matthew Breuer have been using confocal microscopy in their research on novel methods of combatting fungal pathogens.
Among the fungal pathogens they are studying is Cryptococcus neoformans, which is responsible for approximately 181,000 estimated deaths annually. Brain infections from C. neoformans, called cryptococcal meningitis, mainly occur in sub-Saharan Africa.
Sachs said using confocal microscopy has been essential in his research to understand the potential efficacy of sertraline, a commonly prescribed antidepressant, as an antifungal treatment.
“The scarcity of treatments and increasing resistance to current therapeutics highlight the need to develop or identify novel antifungals suitable for clinical use,” he said. “Our previous research has shown sertraline has antifungal properties, and repurposing existing FDA-approved compounds like sertraline as antimycotic therapeutics could be an efficient and effective strategy.”
Sachs and his team used confocal microscopy to obtain higher-resolution images of live Cryptococcus cells and see how they were affected by sertraline.
“These images we obtained in collaboration with Dr. Shaw have been vital in directing our research and knowing where to focus our efforts toward discovering important connections that can help us combat fungal infection,” he said.
In another collaborative effort with Shaw’s lab, ZJ Pei, Ph.D., professor in the College of Engineering Wm Michael Barnes ’64 Department of Industrial and Systems Engineering, and Hongmin Qin, Ph.D., associate professor in the Department of Biology, along with others have used confocal microscopy for research related to bioprinting using plant or algal cells.
“Previous studies on the effects of extrusion pressure and needle diameter on cell viability in bioprinting have used animal cells, but previously there were no reports on how they affected cell viability using plant or algae cells,” Pei said. “Cell quantity is a major indicator of cell viability, and our team used confocal microscopy to measure cell quantity of 3D printed samples.”
The results of “Bioprinting Using Algae: Effects of Extrusion Pressure and Needle Diameter on Cell Quantity in Printed Samples” were printed in the Journal of Manufacturing Science and Engineering, a publication of the American Society of Mechanical Engineers.
“The knowledge about trends of effects of extrusion pressure and needle diameter on algae cell quantity will be beneficial in printing with algae, especially in determining the right extrusion pressure and needle diameter levels,” Pei said.
Shaw and Pei are also collaborating on developing the technology to 3D print fungal-colonized biowastes, such as rice hulls or straw, for use as building materials.
More crucial research on the environmental microbiome
Sanjay Antony-Babu, Ph.D., assistant professor in the Department of Plant Pathology and Microbiology, uses confocal microscopy to learn more about “pathobiome,” or how the microbiome influences soil fungi that cause plant diseases.
Collaborating heavily with Tom Isakeit, Ph.D., a Texas A&M AgriLife Extension Service plant pathologist, Antony-Babu said they are investigating the pathobionts of the fusarium fungus, which causes wilt of various crops, including cotton.
“This plant fungus is a global issue and causes cotton crop damage and losses worldwide,” he said. “We’re looking at what bacteria might be attaching to and helping this pathogenic fungus.”
Antony-Babu said soil microbiomes are highly complex, and it is possible to find more than 10 billion bacteria in one gram of soil.
“No organism in the soil is alone. Only recently are we able to study them as groups rather than individuals,” he said. “Confocal visualization is one major way we study microbiomes.”
Antony-Babu said his team uses a fungal baiting technique where pathogenic fungi recruit the bacteria to which there is a natural attraction.
“We also use a combination of molecular and imaging techniques—DNA/RNA sequencing methods, culture-based analyses and confocal imaging—to find out which organisms are directly interacting with each other to initiate plant disease,” he said.
Shaw also collaborated with Jeanmarie Verchot, Ph.D., a professor in the Department of Plant Pathology and Microbiology whose research focuses on virus-host interactions, using confocal microscopy to document virus replication in plants.
“Fungi are crucial components of the environmental microbiome that is all around us,” Shaw said.
He noted fungi play a role in the nutrient cycle through the degradation of organic waste, as well as in the many diseases affecting plants and animals.
“There’s also the threat of toxic fungal spores degrading air quality in our built environments, so fungi impact all of us daily,” Shaw said.
He said understanding how fungi reproduce and grow in the environment using advanced confocal microscopy is key to human health and prosperity—and a major component of the bioenvironmental sciences.
More information:
Ketan Thakare et al, Bioprinting Using Algae: Effects of Extrusion Pressure and Needle Diameter on Cell Quantity in Printed Samples, Journal of Manufacturing Science and Engineering (2020). DOI: 10.1115/1.4048853
Provided by
Texas A&M University
Citation:
Understanding the environmental microbiome using confocal microscopy (2022, November 29)
retrieved 29 November 2022
from https://phys.org/news/2022-11-environmental-microbiome-confocal-microscopy.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.