Researchers have developed a method to precisely locate hydrogen atoms within nanofilams, a breakthrough with significant implications for superconductivity and other material properties.
Their study, employing nuclear reaction analysis and ion channeling, revealed how hydrogen and its isotopes are distributed within titanium nanofilms, offering insights into tuning the material properties for various applications including hydrogen storage and catalysis.
Impact of Hydrogen on Material Properties
Hydrogen, despite being the smallest and lightest atom, has a significant impact on materials by infiltrating them and altering their properties, such as superconductivity and metal-insulator transitions. Researchers in Japan have now developed a method to more easily pinpoint hydrogen within nanofilms.
In a new study published today (November 14) in Nature Communications, scientists from the Institute of Industrial Science at The University of Tokyo introduced a technique to accurately locate hydrogen atoms in nanofilms.
Hydrogen’s Role in Titanium Nanofilms
Due to their tiny size, hydrogen atoms can migrate into the structure of other materials. For example, titanium absorbs hydrogen to form titanium hydrides, making it valuable for applications like hydrogen storage.
Knowing the precise amount and location of hydrogen atoms is essential for adjusting material properties. However, detecting hydrogen remains difficult because common techniques, like electron probes and X-rays, often lack the sensitivity needed for such small atoms.
Advanced Techniques for Hydrogen Detection
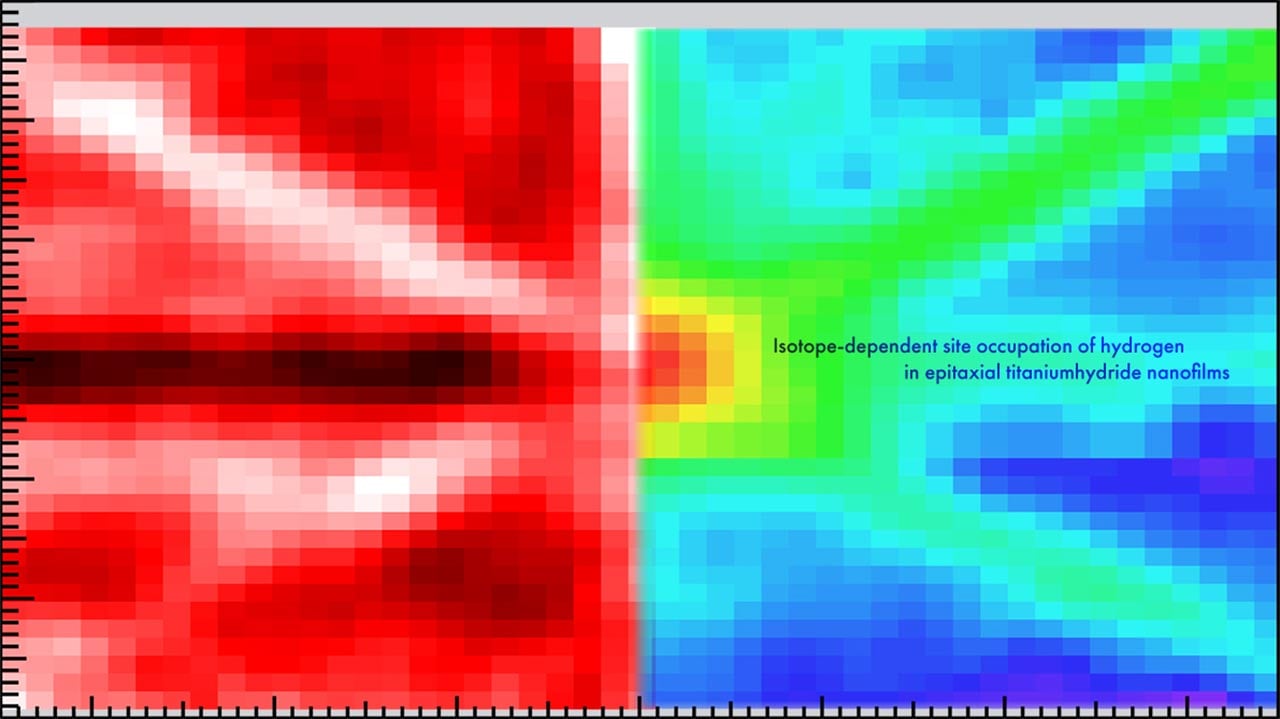
The researchers combined two techniques—nuclear reaction analysis (NRA) and ion channeling—to generate two-dimensional angular mapping of titanium hydride nanofilms.
“We took a close look at a TiH1.47 nanofilm,” explains lead author of the study Takahiro Ozawa. “Understanding nanofilms is useful as many hydrogen-related applications involve surface and subsurface reactions. We were able to precisely locate both the hydrogen and deuterium atoms in the nanofilm.”
All the deuterium atoms—an isotope of hydrogen with double its mass—were at locations in the titanium crystal known as tetrahedral positions. However, 11% of the hydrogen atoms present were at sites described as octahedral. Calculations showed that having this variety in the sites lowered the symmetry, which made the lattice more stable.
Tuning Nanofilm Properties Through Isotope Control
Because the deuterium atoms didn’t occupy octahedral sites because of nuclear quantum effects, controlling the ratio of hydrogen isotopes could be used as a means of tuning the stability and properties of nanofilms based on the intended application.
“Being able to differentiate between the two isotopes in the hydride revealed an opportunity for control,” says Katsuyuki Fukutani, senior author. “This will clearly have important practical applications for producing particular hydrogen-induced phenomena.”
The enhanced understanding of titanium hydride nanofilms is also expected to contribute to hydrogen storage, solid electrolyte, and heterogeneous catalysis applications as we move toward practical and safe green solutions for the future.
Reference: “Isotope-dependent site occupation of hydrogen in epitaxial titanium hydride nanofilms” 14 November 2024, Nature Communications.
DOI: 10.1038/s41467-024-53838-6

