At TU Wien, a study revealed that silver nanoparticles on carbon are 200 times more effective than their pure counterparts due to the interaction at the carbon interface.
This breakthrough could transform industrial catalysis by reducing the precious metal quantity required, lowering costs, and enhancing efficiency.
Catalysts and Their Carbon Base
Precious metals like silver, platinum, and palladium are essential catalysts in the chemical industry. They enable reactions that might otherwise not occur or would happen much more slowly. These metals are often used as tiny nanoparticles, but their effectiveness also depends on the surface they are placed on. Nanoparticles supported on a carbon base have shown particularly strong performance, although the reason for this was unclear for many years.
Researchers at TU Wien recently succeeded in precisely measuring and explaining the interaction between metal nanoparticles and a carbon substrate. They discovered that silver atoms on a carbon support were two hundred times more active than silver atoms in pure form. Computer simulations revealed that the critical factor is the zone where the silver is in direct contact with the carbon. Using hydrogen isotope exchange, the team developed a faster, simpler method for evaluating catalyst supports for effectiveness.
Unveiling the Magic of Carbon in Catalysis
“For a long time, the use of carbon as a carrier material for catalysis had something almost magical,” says Prof. Günther Rupprechter from the Institute of Materials Chemistry at TU Wien. The source of carbon turned out to be important. For some processes, carbon is used that was obtained from coconut shells, fibers, or special woods. Such “recipes” can even be found in patent documents – although the origin of chemical substances should actually be relatively irrelevant. “It always seemed a bit like black art,” says Günther Rupprechter.
The idea was that different manufacturing methods could lead to minimal chemical or physical differences: Perhaps the carbon arranges itself in different ways depending on the method of manufacturing. Maybe it contains traces of other chemical elements? Or do functional groups accumulate on the surface – small molecular building blocks that intervene in the chemical reaction?
“In the chemical industry, people are naturally often content with the fact that a process works and can be repeated reliably,” says Rupprechter. “But we wanted to get to the origin of the effect and understand exactly what is actually going on here at the atomic level.” The University of Cádiz (Spain) and the Center for Electron Microscopy USTEM at TU Wien were also involved.
Microreactor Advances in Catalytic Research
The team first produced samples that could be characterized extremely precisely: silver nanoparticles of a known size on a carbon substrate – and a thin silver foil without carbon.
Both samples were then examined in a chemical reactor: “Silver can be used to split hydrogen molecules into individual hydrogen atoms,” explains Thomas Wicht, the first author of the study. “This hydrogen can then be used, for example, for the hydrogenation reaction of ethene. In an analogous manner, one can also mix ‘ordinary’ hydrogen molecules with molecules made of heavy hydrogen (deuterium). Both molecules are then dissociated by the silver and recombined.” The more active the catalyst, the more frequently the two hydrogen isotopes are exchanged. This provides very reliable information about the catalyst activity.
New Insights into Catalyst Efficiency
This meant that for the first time, the difference in activity between silver atoms with and without a carbon support could be precisely quantified – with spectacular results: “For each silver atom, the carbon background induces a two hundred times higher activity,” says Thomas Wicht. “This is of course very important for industrial applications. You only need a two-hundredth of the amount of expensive precious metals to achieve the same activity – and you can do that simply by adding comparatively inexpensive carbon.”
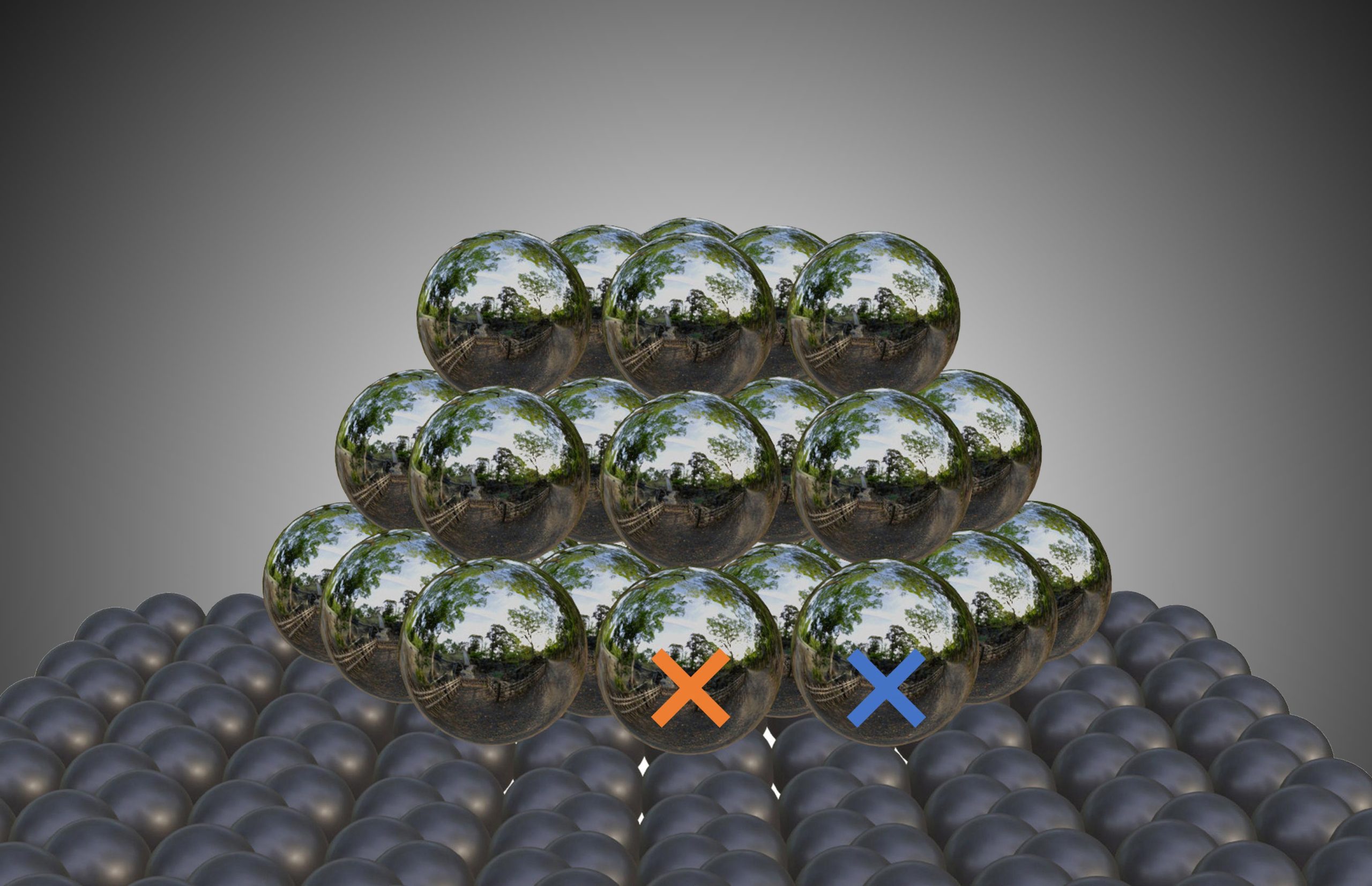
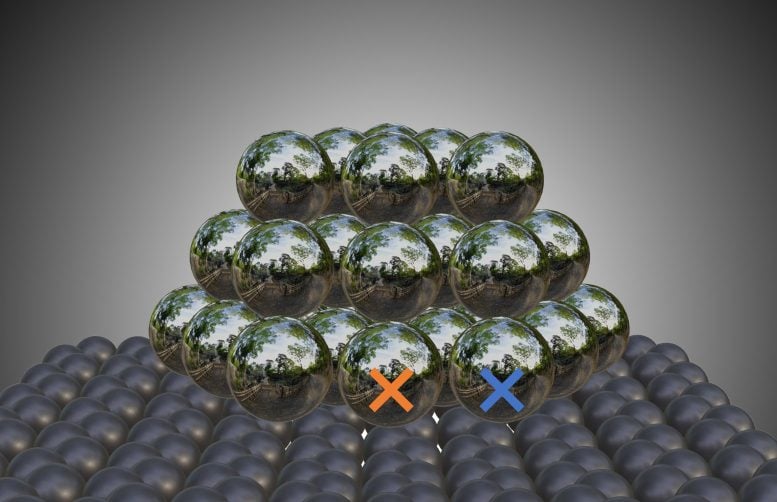
Alexander Genest from the TU Wien team carried out computer simulations comparing the activation of hydrogen by silver nanoparticles on carbon and pure silver. This made it clear: the boundary region between silver particles and carbon carrier is crucial. The catalyst effect is greatest exactly where the two come into contact. “So it’s not the size of the carbon surface or any foreign atoms or functional groups. An extreme catalytic effect occurs when a reactant molecule comes into contact with both a carbon and a silver atom directly at the interface,” says Alexander Genest. The larger this area of direct contact, the greater the activity.
This knowledge means that different carbon batches from different sources can now be checked quite easily for their effectiveness. “Now that we have understood the mechanism of action, we know exactly what to pay attention to,” says Günther Rupprechter. “Our experiment, in which we expose the catalysts to a mixture of ordinary and heavy hydrogen, is relatively easy to carry out and provides very reliable information as to whether this variant of the carbon carrier is also suitable for other chemical reactions or not.” Being able to explain processes at the atomic level should now save time and money in industrial use and simplify quality assurance.
Reference: “Role of Interfacial Hydrogen in Ethylene Hydrogenation on Graphite-Supported Ag, Au, and Cu Catalysts” by Thomas Wicht, Alexander Genest, Lidia E. Chinchilla, Thomas Haunold, Andreas Steiger-Thirsfeld, Michael Stöger-Pollach, José J. Calvino and Günther Rupprechter, 1 November 2024, ACS Catalysis.
DOI: 10.1021/acscatal.4c05246
Research supported by the Austrian Science Fund (FWF; [10.55776/I4434-N and 10.55776/COE5] (Single Atom Catalysis and Cluster of Excellence Materials for Energy Conversion and Storage, MECS).