Scientists design bioinspired hydrogels that mimic plant photosynthesis for clean hydrogen energy production.
A new hydrogel developed by Japanese scientists uses sunlight to efficiently produce hydrogen from water, mimicking natural photosynthesis. This innovation promises to enhance clean energy production by improving efficiency and reducing costs, potentially replacing current technologies with a more sustainable solution.
Artificial Photosynthesis Renewable Energy Technology
Scientists have long aspired to replicate how plants convert sunlight into energy, hoping to create sustainable energy solutions. Artificial photosynthesis, the process of using sunlight to drive clean energy-producing reactions, aims to imitate this natural method. Yet, achieving a synthetic system that functions as smoothly as photosynthesis has been a major challenge—until now.
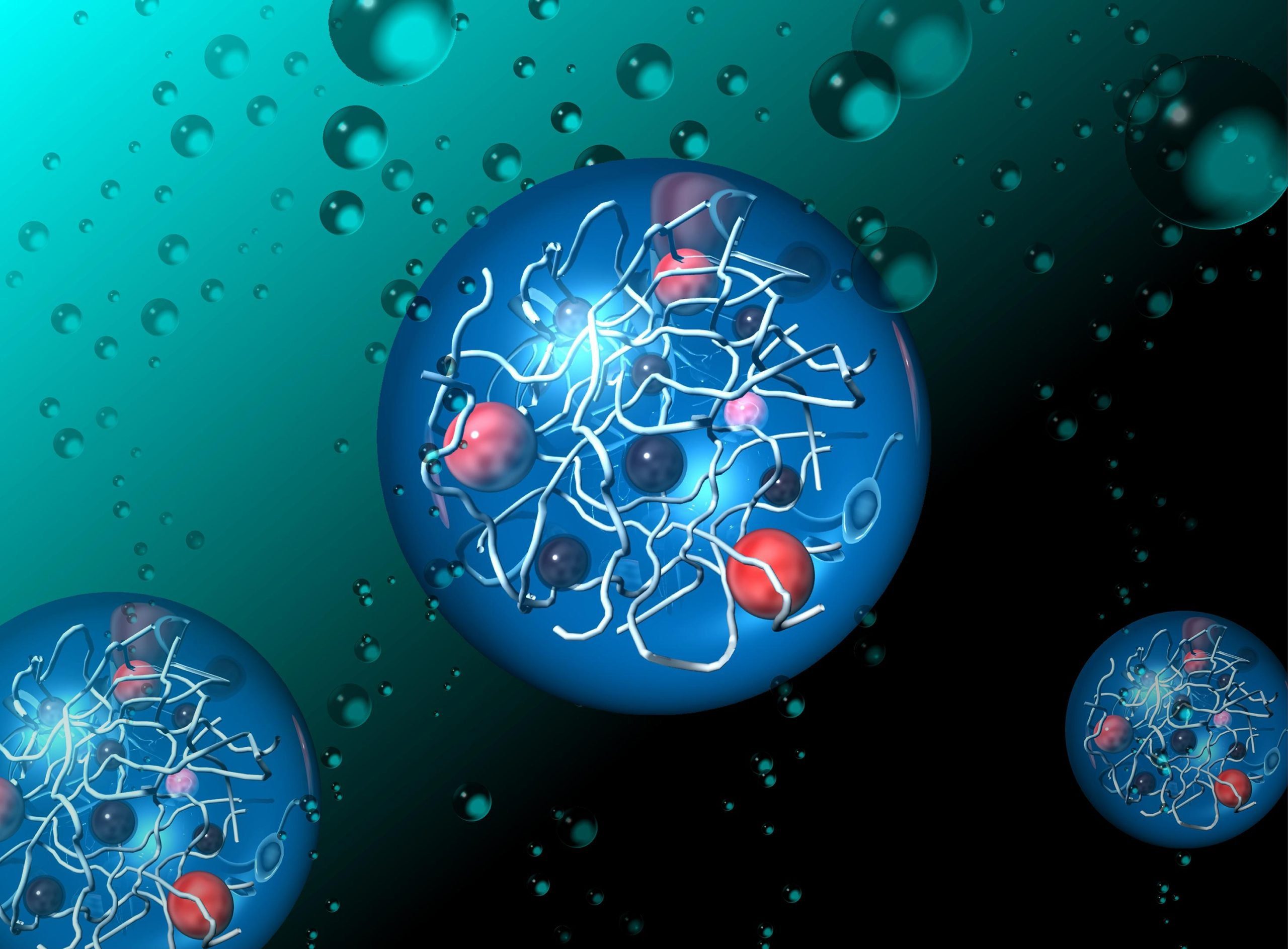
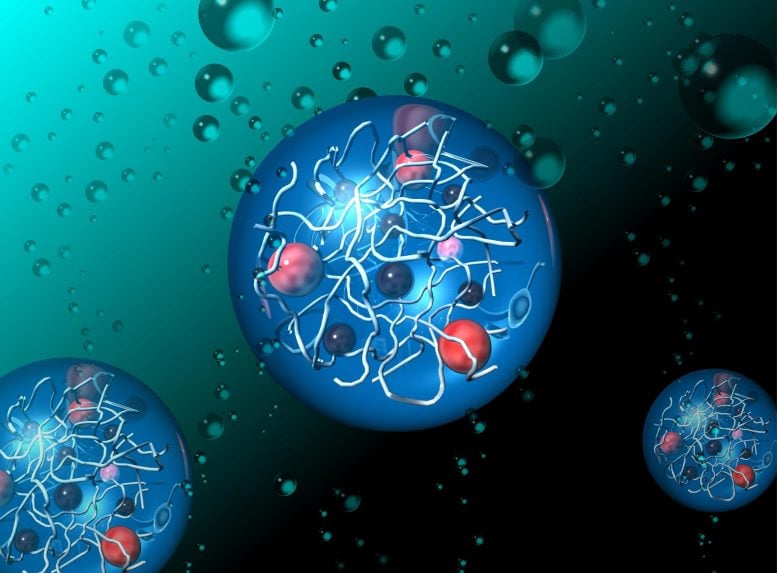
Researchers from the Japan Advanced Institute of Science and Technology (JAIST) and the University of Tokyo have developed a new bioinspired hydrogel capable of generating hydrogen and oxygen by using sunlight to split water molecules. This innovation holds significant promise in the clean energy landscape, with hydrogen viewed as a highly viable fuel for the future. Unlike solar photovoltaics and electrolysis-based hydrogen production, which require external energy inputs, this hydrogel system directly uses sunlight to split water, mirroring nature and potentially increasing both efficiency and cost-effectiveness. The study was recently published online in Chemical Communications.
Design and Mechanics of Bioinspired Hydrogels
The research team, led by Associate Professor Kosuke Okeyoshi, along with his doctoral student Reina Hagiwara at JAIST, and Professor Ryo Yoshida at the University of Tokyo, designed these hydrogels with carefully structured polymer networks. These networks help control the transfer of electrons, which is crucial for splitting water into hydrogen and oxygen. The hydrogels are packed with functional molecules, such as ruthenium complexes and platinum nanoparticles, which work together to simulate the natural process of photosynthesis.
“The biggest challenge was figuring out how to arrange these molecules so they could transfer electrons smoothly,” says Prof. Okeyoshi. “By using a polymer network, we were able to prevent them from clumping together, which is a common issue in synthetic photosynthesis systems.”
Adding further, first-author Reina Hagiwara, a Ph.D. student at JAIST, says, “What’s unique here is how the molecules are organized within the hydrogel. By creating a structured environment, we’ve made the energy conversion process much more efficient.”
Enhancing Hydrogen Production Efficiency
One of the key breakthroughs in this study is the hydrogels’ ability to prevent the functional molecules from aggregating — a major issue in previous artificial photosynthesis systems. As a result, the team was able to significantly boost the activity of the water-splitting process and produce more hydrogen compared to older techniques.
This design has major implications for clean energy. Hydrogen, when produced using just water and sunlight, could become a key player in future energy systems, offering a renewable alternative to fossil fuels. As Prof. Okeyoshi explains, “Hydrogen is a fantastic energy source because it is clean and renewable. Our hydrogels offer a way to produce hydrogen using sunlight, which could help sustainably reshape energy technologies.”
By making artificial photosynthesis more active, this study moves us closer to a future where renewable hydrogen could power industries, transportation, and energy storage systems.
Future Prospects and Challenges
Despite these promising results, the researchers note that there is still work to be done. Scaling up the production of these hydrogels and ensuring their long-term stability will be important next steps. “We have shown the potential, but now we need to refine the technology for industrial use,” says Prof. Okeyoshi. “The possibilities are exciting, and we’re eager to continue pushing forward.”
The team also plans to explore precise integration in the hydrogels to further enhance their energy conversion efficiency. Here’s wishing them luck and success in this endeavor!
Reference: “Bioinspired hydrogels: polymeric designs towards artificial photosynthesis” by Reina Hagiwara, Ryo Yoshida and Kosuke Okeyoshi, 1 November 2024, Chemical Communications.
DOI: 10.1039/D4CC04033C

