

Detecting early-stage lung cancer may become easier thanks to an innovative approach that analyzes exhaled breath.
Researchers developed an ultrasensitive nanoscale sensor capable of detecting isoprene levels in breath, a biomarker for lung cancer. By incorporating platinum-based nanoclusters, the Pt@InNiOx sensor achieved high sensitivity, with a detection threshold of just 2 parts per billion, surpassing previous sensors.
Exhaled Breath as a Diagnostic Tool
Exhaled breath holds valuable chemical clues about our health, including indicators of diseases like lung cancer. Developing ways to detect these compounds could enable doctors to make earlier diagnoses and improve outcomes for patients.
In a study published today (November 6) in ACS Sensors, researchers describe creating ultrasensitive, nanoscale sensors that, in preliminary tests, successfully identified a key chemical change in the breath of individuals with lung cancer. This research arrives timely as November marks Lung Cancer Awareness Month.
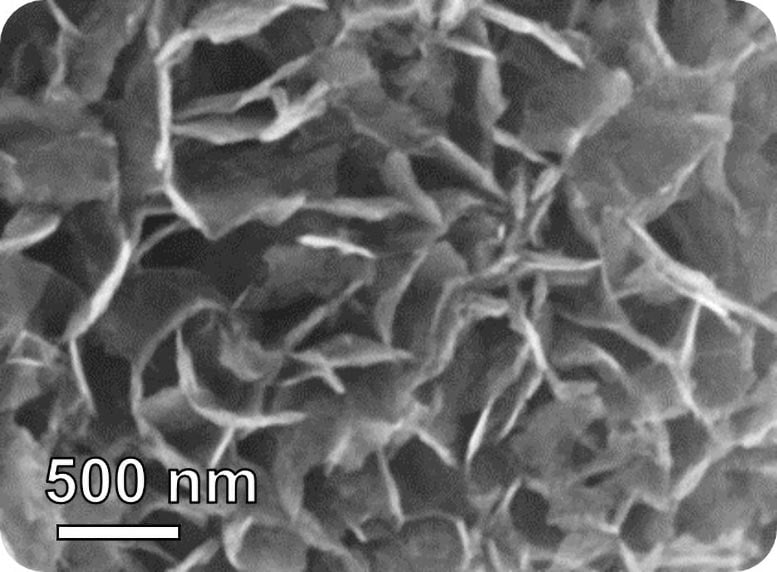
Breath Gases and Their Diagnostic Potential
When we breathe out, we release various gases, including water vapor, carbon dioxide, and other airborne compounds. Among these, researchers have found that reduced levels of a specific chemical, isoprene, may signal lung cancer.
Detecting these small changes requires highly sensitive sensors capable of measuring isoprene levels in the parts-per-billion (ppb) range. Additionally, these sensors must distinguish isoprene from other volatile chemicals in the breath and withstand natural humidity.
Earlier efforts to create such sensors focused on metal oxides, particularly a promising compound made with indium oxide. With this in mind, a research team led by Pingwei Liu and Qingyue Wang aimed to refine indium oxide-based sensors to detect isoprene at the levels naturally found in breath.
Advancing Sensor Sensitivity With Indium Oxide
The researchers developed a series of indium(III) oxide (In2O3)-based nanoflake sensors. In experiments, they found one type, which they called Pt@InNiOx for the platinum (Pt), indium (In), and nickel (Ni) it contains, performed best. These Pt@InNiOx sensors:
- Detected isoprene levels as low as 2 ppb, a sensitivity that far surpassed earlier sensors.
- Responded to isoprene more than other volatile compounds commonly found in breath.
- Performed consistently during nine simulated uses.
More importantly, the authors’ real-time analysis of the nanoflakes’ structure and electrochemical properties revealed that Pt nanoclusters uniformly anchored on the nanoflakes catalyzed the activation of isoprene sensing, leading to ultrasensitive performance.
Portable Device Development for Real-World Testing
Finally, to showcase the potential medical use of these sensors, the researchers incorporated the Pt@InNiOx nanoflakes into a portable sensing device. Into this device, they introduced breath collected earlier from 13 people, five of whom had lung cancer. The device detected isoprene levels lower than 40 ppb in samples from participants with cancer and more than 60 ppb from cancer-free participants. This sensing technology could provide a breakthrough in non-invasive lung cancer screening and has the potential to improve outcomes and even save lives, the researchers say.
Reference: “Ultrasensitive In2O3‑Based Nanoflakes for Lung Cancer Diagnosis and the Sensing Mechanism Investigated by Operando Spectroscopy” 6 November 2024, ACS Sensors.
DOI: 10.1021/acssensors.4c01298
The authors acknowledge funding from the National Natural Science Foundation of China, China’s State Key Laboratory of Chemical Engineering, the State Key Laboratory of Electrical Insulation and Power Equipment, the Shanxi-Zheda Institute of Advanced Materials and Chemical Engineering, the National Key Research and Development Program of China-Young Scientists, the Research Funds of Institute of Zhejiang University-Quzhou, and the Science and Technology Program of the Institute of Zhejiang University-Quzhou.