Researchers discovered that ovarian tumors hinder T cells’ energy supply by trapping a key protein, blocking lipid uptake. A new approach to reprogram T cells could enhance immunotherapy for aggressive cancers.
Researchers at Weill Cornell Medicine have uncovered a mechanism by which ovarian tumors weaken immune cells, effectively blocking their energy source and hindering their ability to launch an attack. Published on October 23 in Nature, this discovery offers a potential new immunotherapy strategy for ovarian cancer, a notoriously aggressive and challenging disease to treat.
A significant obstacle in treating ovarian cancer is the tumor microenvironment—the complex ecosystem of cells, molecules and blood vessels that shields cancer cells from the immune system. Within this hostile environment, T cells lose their ability to take up the lipid (fat) molecules, which are necessary for energy to mount an effective attack.
“T cells rely on lipids as fuel, burning them in their mitochondria to power their fight against pathogens and tumors,” explained senior author, Dr. Juan Cubillos-Ruiz, The William J. Ledger, M.D., Distinguished Associate Professor of Infection and Immunology in Obstetrics and Gynecology at Weill Cornell Medicine. “However, the molecular mechanisms that govern this critical energy supply are still not well understood.”
Identifying How Tumors Block T-Cell Energy Supply
Lipids are abundant in ovarian tumors, but T cells seem unable to utilize them in this environment. “Researchers have focused on a protein called fatty acid-binding protein 5, or FABP5, which facilitates lipid uptake, but its exact location within the T cell remained unclear,” said Dr. Sung-Min Hwang, a postdoctoral associate in Dr. Cubillos-Ruiz’s lab who led the new study. Dr. Hwang discovered that in patient-derived tumor specimens and mouse models of ovarian cancer, FABP5 becomes trapped inside the cytoplasm of T cells instead of moving to the cell surface, where it would normally help take up lipids from the surroundings.
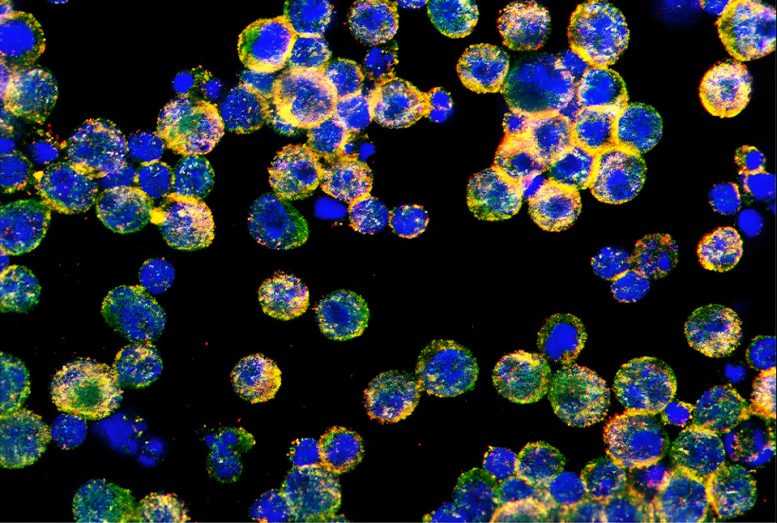
“That was the ‘aha!’ moment; since FABP5 is not getting to the surface, it couldn’t bring in the lipids necessary for energy production. But we still needed to figure out why,” said Dr. Cubillos-Ruiz, who is also co-leader of the Cancer Biology Program in the Sandra and Edward Meyer Cancer Center at Weill Cornell Medicine.
Working with collaborators, the researchers used a battery of biochemical assays to identify proteins that bind to FABP5. They found a protein called Transgelin 2 that interacts with FABP5 and helps move it to the cell surface.
Further experiments revealed that ovarian tumors suppress the production of Transgelin 2 in infiltrating T cells. Delving deeper, the researchers discovered that the transcription factor XBP1, which is activated by the stressful conditions within the tumor, represses the gene encoding Transgelin 2. Without Transgelin 2, FABP5 is trapped in the cytoplasm of T cells, preventing lipid uptake and rendering the T cells unable to attack the tumor.
Designer Immunotherapies to Overcome Tumor Defenses
With this fundamental mechanism worked out, the team explored an immunotherapy called chimeric antigen receptor T (CAR T) cells. This approach collects a patient’s T cells, engineers them to attack tumor cells and then injects the designer cells into the patient. “CAR T cells work well against hematological cancers like leukemia and lymphoma, but they’re really not effective for solid tumors like ovarian or pancreatic cancers,” Dr. Cubillos-Ruiz said.
When Dr. Hwang and his colleagues tested CAR T cells, which are currently being evaluated in clinical trials, in mouse models of metastatic ovarian cancer, they found the same problem—Transgelin 2 repression and impaired lipid uptake. Just like normal T cells in the tumor microenvironment, the engineered CAR T cells had FABP5 tangled in the cytoplasm. As a result, the CAR T cells were unable to access lipids for energy to effectively attack the tumor, highlighting a critical barrier in using this immunotherapy for solid tumors like ovarian cancer.
To solve the problem, the researchers inserted a modified Transgelin 2 gene that couldn’t be blocked by stress transcription factors, so expression of the critical protein was preserved. This allowed Transgelin 2 to chaperone FABP5 to the surface of the CAR T cells where it could take up lipids.
Indeed, the upgraded T cells were much more effective in attacking ovarian tumors than the original CAR T cells. “Our findings reveal a key mechanism of immune suppression in ovarian cancer and suggest new avenues to improve the efficacy of adoptive T cell immunotherapies in aggressive solid malignancies,” Dr. Cubillos-Ruiz said.
Reference: “Transgelin 2 guards T cell lipid metabolism and antitumour function” by Sung-Min Hwang, Deepika Awasthi, Jieun Jeong, Tito A. Sandoval, Chang-Suk Chae, Yusibeska Ramos, Chen Tan, Matías Marin Falco, Camilla Salvagno, Alexander Emmanuelli, Ian T. McBain, Bikash Mishra, Lionel B. Ivashkiv, Dmitriy Zamarin, Evelyn Cantillo, Eloise Chapman-Davis, Kevin Holcomb, Diana K. Morales, Xiaoqing Yu, Paulo C. Rodriguez, Jose R. Conejo-Garcia, Martin Kaczocha, Anna Vähärautio, Minkyung Song and Juan R. Cubillos-Ruiz, 23 October 2024, Nature.
DOI: 10.1038/s41586-024-08071-y
This work was supported in part by the National Institutes of Health grants R01 NS114653, CA271619, CA282072, R01 CA237154 and R01 CA269382, the U.S. Department of Defense grants W81XWH2010191, W81XWH-16-1-0438, W81XWH-22-OCRP-IIRA, W81XWH2110478 and W81XWH2110357, and the American Association for Cancer Research; AACR-Bristol Myers Squibb Immuno-Oncology Research Fellowship.