Researchers have identified a key mechanism in the development of Alzheimer’s disease involving the growth and pause of amyloid β fibrils.
A newly discovered antibody can lock these fibrils in their paused state, offering a potential new approach for treatment that targets these critical growth points.
Breakthrough in Alzheimer’s Research
A team of researchers from the Exploratory Research Center on Life and Living Systems, the Institute for Molecular Science, and several universities in Japan—including Nagoya City University, Nagoya University, and the University of Tsukuba—has uncovered a new mechanism behind the growth of amyloid β (Aβ) fibrils, which are closely linked to Alzheimer’s disease. Using advanced high-speed atomic force microscopy (HS-AFM), they observed Aβ fibril growth in real time at the molecular level. This discovery provides fresh insight into how these fibrils form and reveals potential ways to stop their progression.
Alzheimer’s disease is a debilitating neurodegenerative disorder that causes memory loss and cognitive decline. One of its key drivers is the buildup of Aβ proteins in the brain, which clump together to form fibrils that disrupt normal brain function. Understanding how these fibrils grow and how their formation can be halted is critical to developing new treatments, but until now, the specific mechanisms behind their growth have been elusive.
Insights Into Fibril Growth Mechanism
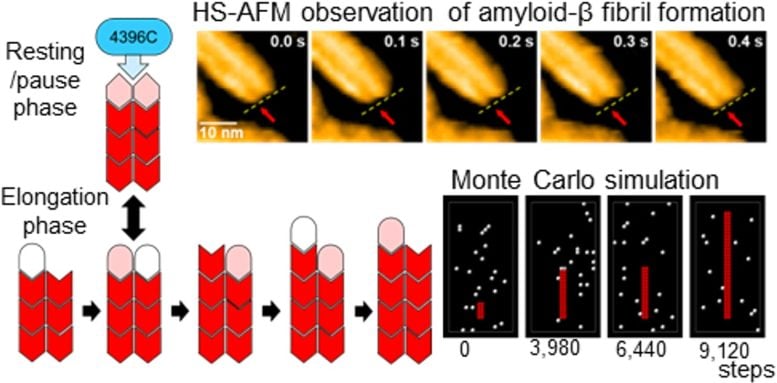
The researchers found that each Aβ fibril is composed of two thin strands, called protofilaments. These protofilaments grow in an alternating pattern, with individual Aβ molecules adding to the ends of each strand one at a time. A critical finding of the study was that when the ends of these two protofilaments align, the fibril enters a “paused state,” where growth temporarily stops. This pause in growth is a crucial step in the Aβ fibril formation process and could be key to understanding how Alzheimer’s disease progresses.
One of the most notable discoveries was the role of an antibody, 4396C, which selectively binds to the ends of the Aβ fibrils during this paused state. Once the antibody binds, the fibril is locked in this state, and further growth is prevented. This finding reveals a promising new approach to stopping Aβ fibril growth and, potentially, slowing the progression of Alzheimer’s disease.
Real-time HS-AFM observation of Aβ fibril growth and the 4396C antibody binding at the fibril end. Credit: Koichi Kato and Takayuki Uchihashi
Future Implications and Therapeutic Prospects
The detailed high-resolution observations made with HS-AFM allowed the research team to uncover this alternating growth and pause mechanism, which had not been previously identified. By targeting the paused state of Aβ fibrils, this study opens up new possibilities for developing treatments that can delay or halt Alzheimer’s disease at the molecular level.
In the future, the team plans to further investigate the action of the 4396C antibody, with the hope of applying these findings to create new therapeutic approaches for Alzheimer’s disease. Additionally, the discovery may have broader implications for other amyloid-related diseases, as the insights gained from this study could inform treatments for a range of conditions involving protein aggregation.
Reference: “Single-molecule kinetic observation of antibody interactions with growing amyloid β fibrils” 24 October 2024, Journal of the American Chemical Society.
DOI: 10.1021/jacs.4c08841