

Research paves the way for new advancements in clean energy production.
Amid the ongoing energy transition and the battle against climate change, a study from the Department of Energy at Politecnico di Milano introduces innovative methods for utilizing greenhouse gases. Featured prominently on the cover of the renowned scientific journal Angewandte Chemie, the research provides new insights into improving the efficiency of processes that transform greenhouse gases into energy resources, while also mitigating the impact of methane and CO2—two major contributors to global warming.
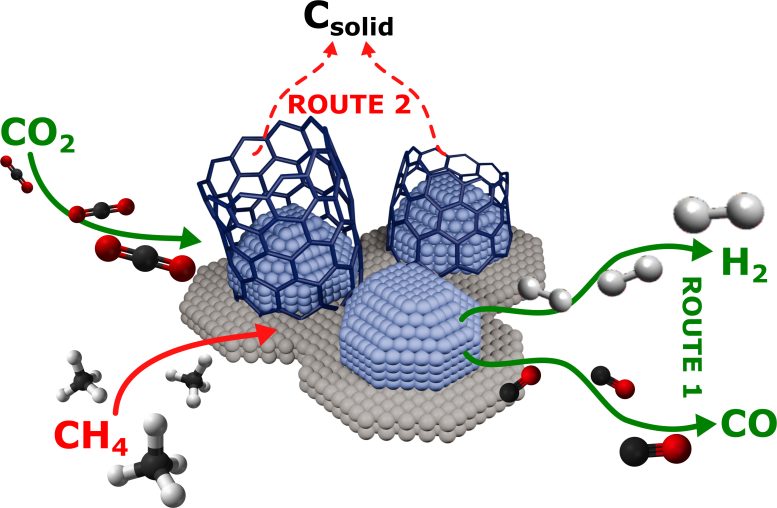
The research team headed by Prof. Matteo Maestri studied Dry Reforming, a chemical process that converts methane and carbon dioxide, two of the main greenhouse gases, into a synthesis gas that is used both in hydrogen production and in many sectors of the chemical and energy industries. Using supported metal nanoparticles as catalysts, the Dry Reforming process enables high conversions, accelerating the necessary chemical reactions.
Challenges: Carbon Build-Up on Catalysts
However, one of the main obstacles to a more widespread application of this process is the build-up of carbon on the surface of the catalysts, a phenomenon that reduces their efficiency and makes them less suitable for large-scale use. Using operando Raman spectroscopy, an advanced technique that allows catalysts to be studied in real-time during chemical reactions, the team discovered that the gradual formation of carbon is closely related to the ratio of carbon dioxide (CO2) to methane (CH4) present in the reaction.
“Our work allowed us to observe how a catalyst transforms during the reaction,’ explains Prof. Matteo Maestri from the Department of Energy at Politecnico di Milano “Knowing this will help us improve the efficiency of catalysts, with a potentially significant impact on the reduction of greenhouse gas emissions and long-term energy sustainability.”
The possibility of preventing or mitigating carbon build-up on catalysts paves the way for longer-lasting and more efficient technologies based on this reaction, offering new solutions for the use of CO2 biogas.
Reference: “Surface Carbon Formation and its Impact on Methane Dry Reforming Kinetics on Rhodium-Based Catalysts by Operando Raman Spectroscopy” by Riccardo Colombo, Gianluca Moroni, Chiara Negri, Guusje Delen, Matteo Monai, Alessandro Donazzi, Bert M. Weckhuysen and Matteo Maestri, 03 July 2024, Angewandte Chemie International Edition.
DOI: 10.1002/anie.202408668