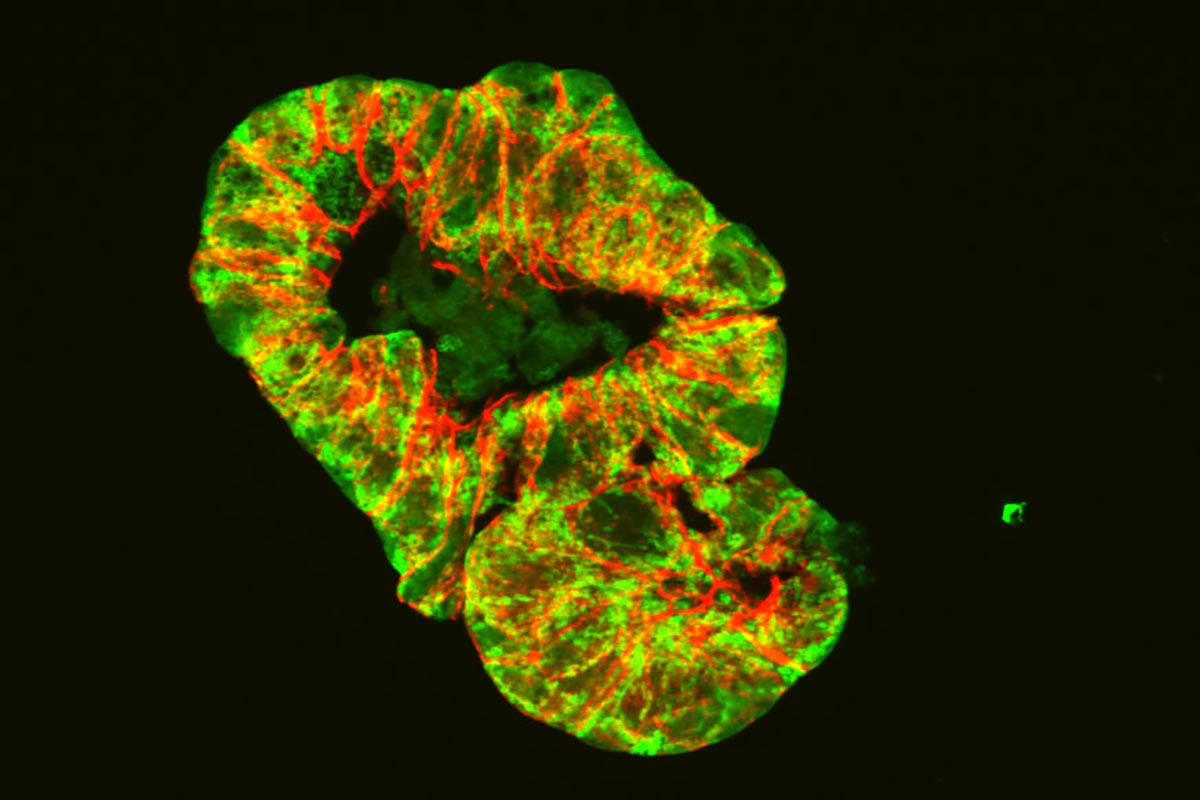
Researchers at the Technical University of Munich (TUM) have linked mitochondrial dysfunctions to Crohn’s disease, demonstrating that defective mitochondria in mice can induce symptoms of chronic intestinal inflammation and affect the microbiome. This finding opens up possibilities for new treatments focused on mitochondrial restoration.
Common symptoms of Crohn’s disease (CD) include chronic diarrhea, abdominal pain, and fever. Although the causes of Crohn’s disease remain poorly understood, it has been known for years that changes in the gut microbiome—the community of microbes residing in the intestinal tract—are associated with inflammatory diseases. Some researchers propose that these changes, the causes of which remain unknown, may trigger the disease.
A team led by Dirk Haller, Chair of Nutrition and Immunology and Director of the Institute for Food and Health at the Technical University of Munich TUM (ZIEL), has searched for the causes of these changes in the microbiome and investigated the interplay of the microbiome, the intestinal epithelium, and mitochondria. The intestinal epithelium is a cell layer that lines the inside of the intestine, absorbs nutrients, and fights off pathogens. Mitochondria are small cellular structures that convert nutrients into energy and therefore influence cellular metabolism and the ability of cells to perform their functions.
Mitochondria Disruption Leads to Changes in the Microbiome
Haller and his team have been pursuing the hypothesis that mitochondria not only serve as the power plants of cells but also interact with the microbiome. In addition, previous research has shown that the intestinal epithelium in patients with chronic intestinal inflammation exhibits certain stress markers indicating possible mitochondrial malfunction.

For their study, the researchers therefore disrupted mitochondrial function in mice by deleting a gene segment responsible for producing the protein Hsp60. This protein is essential to the ability of mitochondria to perform their tasks. The intervention triggered various processes in the gut. For one, tissue injuries were identified in the intestinal epithelium similar to those seen in Crohn’s disease patients. Changes were also seen at the level of gene activation that is typical of some stages of the disease. In addition – an essential development for the question investigated by the team – the microbiome responded to the disrupted mitochondria by changing its composition.
As a result, Haller and his team were able to demonstrate for the first time that disruptions to mitochondria are causally related to tissue damage in the intestines and also trigger disease-related changes in the microbiome.
Prospects for New Drugs
This insight may prove important to persons with inflammatory conditions because it presents potential approaches for new treatments. Currently, treatment is limited to alleviating the symptoms of the disease with anti-inflammatory medications. “The big hope is to find active ingredients that would restore the functionality of disrupted mitochondria, in other words to repair them in a sense. This would limit intestinal damage as a trigger for chronic inflammation processes,” says Haller. “Our results suggest that drugs that act on mitochondrial pathways or address the connections between the microbiome and mitochondria could be a key aspect of better treatments.”
Reference: “Mitochondrial perturbation in the intestine causes microbiota-dependent injury and gene signatures discriminative of inflammatory disease” by Elisabeth Urbauer, Doriane Aguanno, Nora Mindermann, Hélène Omer, Amira Metwaly, Tina Krammel, Tim Faro, Marianne Remke, Sandra Reitmeier, Stefanie Bärthel, Johannes Kersting, Zihua Huang, Feng Xian, Manuela Schmidt, Dieter Saur, Samuel Huber, Bärbel Stecher, Markus List, David Gómez-Varela, Katja Steiger, Matthieu Allez, Eva Rath and Dirk Haller, 15 July 2024, Cell Host & Microbe.
DOI: 10.1016/j.chom.2024.06.013