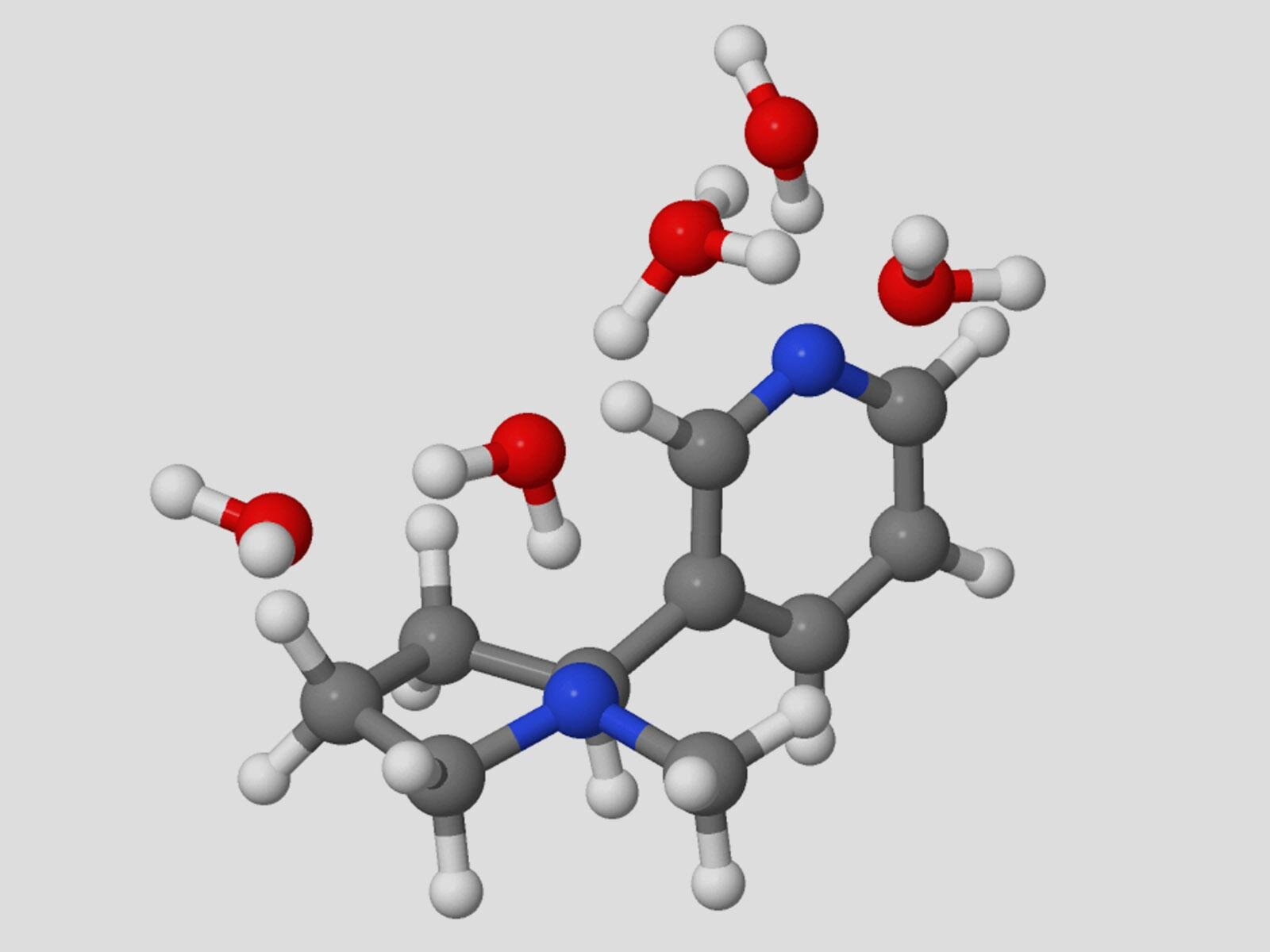
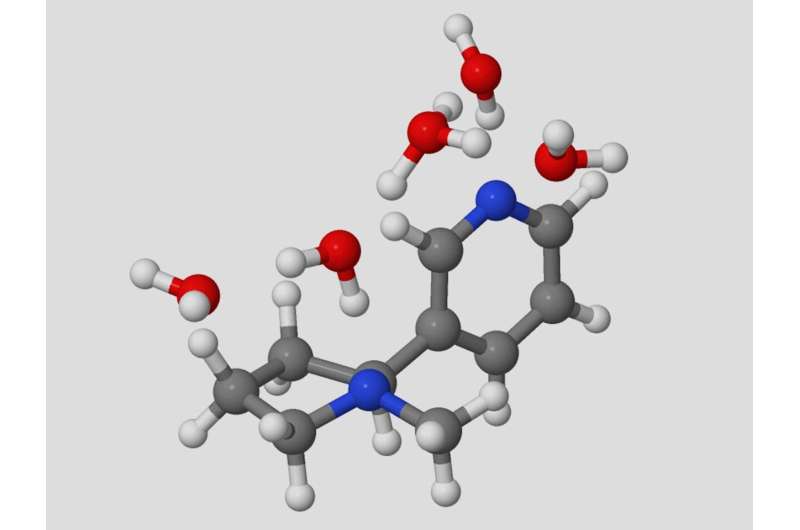
Proton transfer is central to numerous important systems, from biology to energy storage. Probing the details of proton transfer reactions can provide insight into how to control these processes.
Nicotine has two potential protonation sites, one of which results in the bioactive, addictive form of the molecule. Researchers probed the proton switching of nicotine in water by infrared spectroscopy and theoretical ab initio calculations.
They found that the proton transfer is facilitated via a Grotthuss instead of a bimolecular vehicle mechanism at 130 K, which was unambiguously confirmed with deuterated water experiments. In contrast, the bimolecular vehicle mechanism is preferred at higher temperatures (T = 300 K), as determined by theory.
Their results are published in the Journal of the American Chemical Society.
The Grotthuss mechanism for concerted proton transfer results in the production of the bioactive and addictive pyrrolidine protonated nicotine protomer with just five water molecules. Theoretical analysis suggests that proton transfer occurs via hydrogen-bonded bridges consisting of a three-water molecule “core” that connects the pyridine and pyrrolidine protomers.
Additional water molecules attach as acceptors to the hydrogen-bonded “core” bridge, lowering the reaction barrier of the concerted proton transfer down to less than 6 kcal/mol, a value that is consistent with experimental observations.
Beyond nicotine, this work provides an example of the power of combining experimental and theoretical approaches in identifying proton transfer mechanisms.
More information:
Yuika Okura et al, Switching of Protonation Sites in Hydrated Nicotine via a Grotthuss Mechanism, Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.3c08922
Provided by
Pacific Northwest National Laboratory
Citation:
Observing the mechanism of protonation site switching in hydrated nicotine (2024, October 14)
retrieved 15 October 2024
from https://phys.org/news/2024-10-mechanism-protonation-site-hydrated-nicotine.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.