Results of the first phase of the Ceramide Ring Trial have been published in Nature Communications, representing a significant landmark in the field of lipidomics.
This achievement, involving researchers at the University of Vienna and scientific teams in Singapore, Julich and Espoo, represents an advance in the establishment of ceramide reference values, plasma lipids involved in diseases such as cardiovascular diseases. The ring trial was performed under the umbrella of the International Lipidomics Society (ILS).
Lipidomics—the large-scale study of pathways and networks of cellular lipids in biological systems—aims to understand the roles of lipids in health and disease by analyzing their structures, functions, and interactions in cells. Understanding the upper and lower concentration boundaries of lipids is essential for scientific progress and the translation of technology within lipidomics.
To do this, the Ceramide Ring Trial was initiated as the first step in addressing technical replication across a global network of laboratories.
A ring trial is a method where multiple laboratories independently analyze the same samples using similar or different methods to compare their results. It helps assess the reliability and consistency of measurements across different labs, improving standardization and quality control in scientific testing.
After seven years of collaborative efforts, the results from 34 participating laboratories across 19 countries have been summarized in the Ceramide Ring Trial study.
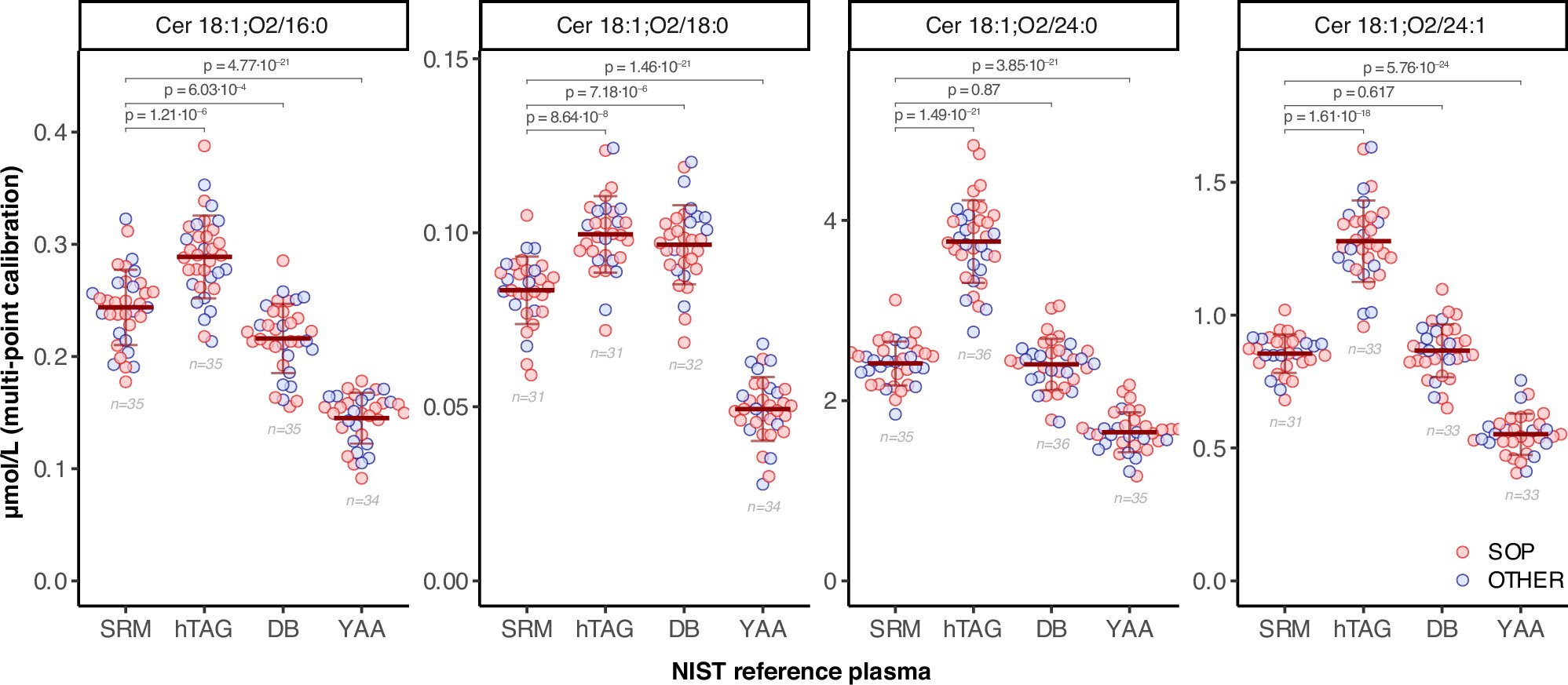
To maintain a strict focus while reducing complexity, the Ceramide Ring Trial concentrated on human plasma/serum and aimed to investigate concentration levels and their variabilities of four distinct ceramide lipid species.
Ceramides are players in multiple diseases and have been associated as biomarkers in cardiovascular diseases. Participants of the trial used a preferred analytical method and/or a standardized protocol to quantify ceramides in NIST1950 (a standard reference material of metabolites in human plasma provided by the National Institutes of Standards and Technology, NIST) and three additional NIST pooled plasma reference materials, by using specially formulated mixtures of ceramide standards from Avanti Polar Lipids.
“A number of valuable lessons can be learned from the results of our interlaboratory comparison,” explains Robert Ahrends from the Department of Analytical Chemistry at the University of Vienna and corresponding author of the study.
- Standardizing is key to reduce variations in the test procedure and reach consensus in the concentrations of the analytes of interest.
- Obtaining mean absolute concentration levels of ceramides sets the baseline for future biological and medical studies relevant to ceramide-associated diseases.
- By comparing mixed plasma samples, the study estimates the biological differences between healthy people, those with high cholesterol, and different ethnic groups.
“This study, the largest and most targeted public inter-laboratory and cross-platform ring trial for distinct ceramides in human plasma, sets a new benchmark for future harmonization of lipidomics research and beyond. We extend our deepest gratitude and congratulations to all participants involved in this pioneering effort,” Ahrends says.
More information:
Federico Torta et al, Concordant inter-laboratory derived concentrations of ceramides in human plasma reference materials via authentic standards, Nature Communications (2024). DOI: 10.1038/s41467-024-52087-x
Provided by
University of Vienna
Citation:
Trial results set benchmark for future clinical applications of lipidomic technologies (2024, October 10)
retrieved 10 October 2024
from https://phys.org/news/2024-10-trial-results-benchmark-future-clinical.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.