New research shows astrocytes can remove Alzheimer’s-related amyloid-beta via autophagy, offering a promising new direction for treatment strategies focusing on these brain cells.
An international research team has identified a new mechanism involving astrocytes for treating Alzheimer’s disease (AD) and proposed a novel therapeutic target. In their study, the researchers revealed that the autophagy pathway in astrocytes (non-neuronal cells in the brain) removes amyloid-beta (Aβ) oligomers, the toxic proteins found in the brains of AD patients, and recovers memory and cognitive functions.
The research, led by Dr. Hoon Ryu from the Korea Institute of Science and Technology (KIST, President Sang-Rok Oh) Brain Disease Research Group, in collaboration with Director Justin C. Lee of the Institute for Basic Science (IBS, President Do-Young Noh) and Professor Junghee Lee from Boston University Chobanian & Avedisian School of Medicine, was published in the journal Molecular Neurodegeneration.
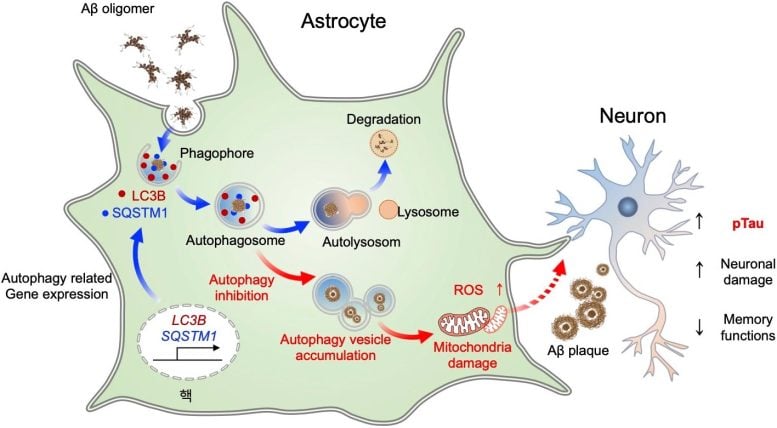
Astrocytes’ Role in Neurodegeneration
AD, a representative form of senile dementia, occurs when toxic proteins like Aβ, abnormally aggregate and accumulate in the brain, leading to inflammation and damage to neurons, causing neurodegenerative disorders. Although the scientific community has long focused on the role of astrocytes in removing toxic proteins around neurons, the exact mechanism remains unclear.
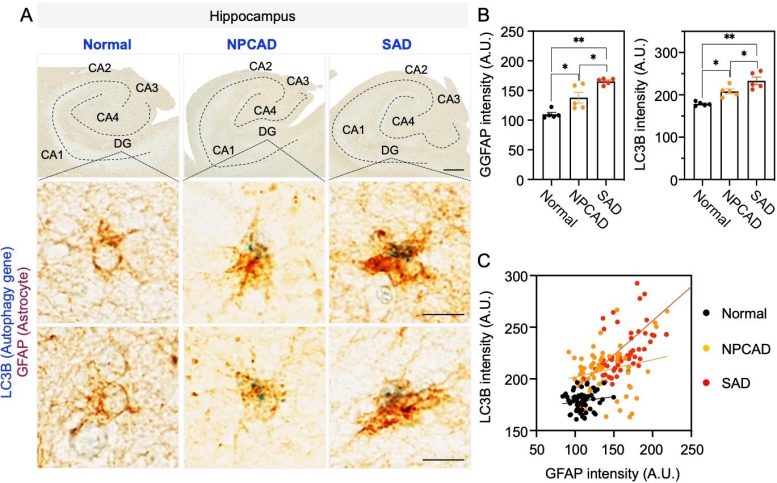
Discoveries in Astrocytic Autophagy
Autophagy is a process by which cells break down and recycle their own components to maintain homeostasis. The research team scrutinized the autophagy process in astrocytes and discovered that when toxic protein buildup or inflammation occurs in the brains of AD patients, astrocytes respond by inducing genes that regulate autophagy. By delivering these autophagy-associated genes specifically into astrocytes in AD mouse models, the researchers observed the recovery of damaged neurons.
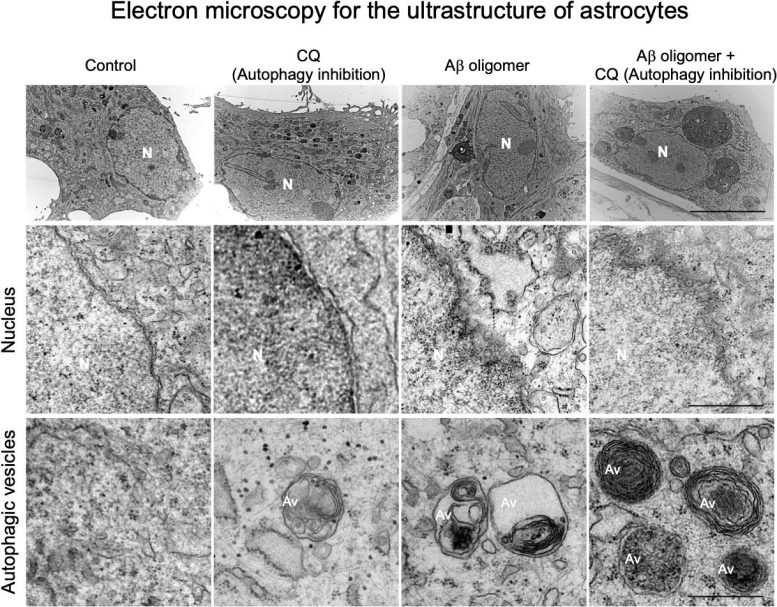
Potential Therapeutic Implications
This study demonstrated that astrocytic autophagy reduces Aβ aggregates (protein clumps) and improves memory and cognitive functions. Notably, when autophagy-associated genes were expressed in astrocytes of the hippocampus, a brain region responsible for memory, the neuropathological symptoms were decreased. Most significantly, this study showed that the autophagy plasticity of astrocytes is involved in eliminating Aβ oligomers, a major cause of AD pathology, thus presenting a new potential therapeutic avenue for treating AD.
Future Directions and Impacts
This research is particularly meaningful as it shifts away from the traditional neuron-centered approach in AD drug development, instead identifying astrocytes (non-neuronal cells) as a novel target for therapy. The research team plans to further explore drug developments that can enhance the autophagic function of astrocytes to prevent or alleviate dementia symptoms and to conduct preclinical studies in the near future.
Dr. Ryu and Dr. Suhyun Kim (the first author) commented, “Our findings show that astrocytic autophagy restores neuronal damage and cognitive functions in the dementia brain. We hope this study will advance our understanding of cellular mechanisms related to autophagy and contribute to future research on waste removal by astrocytes and health maintenance of the brain.”
Reference: “Astrocytic autophagy plasticity modulates Aβ clearance and cognitive function in Alzheimer’s disease” by Suhyun Kim, Heejung Chun, Yunha Kim, Yeyun Kim, Uiyeol Park, Jiyeon Chu, Mridula Bhalla, Seung-Hye Choi, Ali Yousefian-Jazi, Sojung Kim, Seung Jae Hyeon, Seungchan Kim, Yeonseo Kim, Yeon Ha Ju, Seung Eun Lee, Hyunbeom Lee, Kyungeun Lee, Soo-Jin Oh, Eun Mi Hwang, Junghee Lee, C. Justin Lee and Hoon Ryu, 23 July 2024, Molecular Neurodegeneration.
DOI: 10.1186/s13024-024-00740-w
This research was supported by the Ministry of Science and ICT (Minister Sang Im Yoo), under KIST’s Major Projects and the Mid-career Researcher Support Program (2022R1A2C3013138), and the Ministry of Health and Welfare (Minister Gyu-Hong Cho), under the Dementia Overcoming Program (RS-2023-KH137130).