Researchers have captured the first images of a brain network designed to clear waste, potentially staving off diseases like Alzheimer’s.
This glymphatic system was imaged in patients undergoing surgery, confirming theories previously only seen in animal models. The study highlights the system’s efficacy in transporting metabolic byproducts, including harmful proteins, out of the brain via perivascular spaces, suggesting that lifestyle changes could boost this natural protective mechanism.
Groundbreaking Brain Pathway Imaging
Scientists have long speculated on the existence of a network of pathways in the brain designed to clear metabolic proteins that might otherwise accumulate and lead to Alzheimer’s and other forms of dementia. However, this network had never been definitively observed in humans — until now.
A new study involving five patients undergoing brain surgery at Oregon Health & Science University (OHSU) has provided the first images of this network of perivascular spaces — fluid-filled structures along arteries and veins within the brain.
First Definitive Evidence of Glymphatic System
“Nobody has shown it before now,” said senior author Juan Piantino, M.D., associate professor of pediatrics (neurology) in the OHSU School of Medicine and a faculty member of the Neuroscience Section of the Papé Family Pediatric Research Institute at OHSU. “I was always skeptical about it myself, and there are still a lot of skeptics out there who still don’t believe it. That’s what makes this finding so remarkable.”
The study was published today (October 7) in the Proceedings of the National Academy of Sciences.
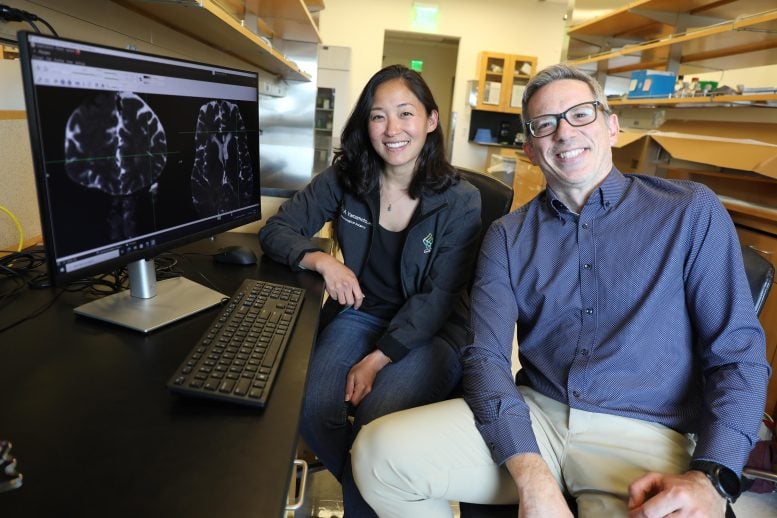
Imaging Techniques and Findings
The study combined the injection of an inert contrasting agent with a special type of magnetic resonance imaging to discern cerebrospinal fluid flowing along distinct pathways in the brain 12, 24, and 48 hours following surgery. In definitively revealing the presence of an efficient waste-clearance system within the human brain, the new study supports the promotion of lifestyle measures and medications already being developed to maintain and enhance it.
“This shows that cerebrospinal fluid doesn’t just get into the brain randomly, as if you put a sponge in a bucket of water,” Piantino said. “It goes through these channels.”
More than a decade ago, scientists at the University of Rochester first proposed the existence of a network of waste-clearance pathways in the brain akin to the body’s lymphatic system, part of the immune system. Those researchers confirmed it with real-time imaging of the brains of living mice. Due to its dependence on glial cells in the brain, they coined the term “glymphatic system” to describe it.
However, scientists had yet to confirm the existence of the glymphatic system through imaging in people.
Confirmation of Glymphatic Function in Humans
The new study examined five OHSU patients who underwent neurosurgery to remove tumors in their brains between 2020 and 2023. In each case, the patients consented to having a gadolinium-based inert contrasting agent injected through a lumbar drain used as part of the normal surgical procedure for tumor removal. The tracer would be carried with cerebrospinal fluid into the brain.
Afterward, each patient underwent magnetic resonance imaging, or an MRI, at different time points to trace the spread of cerebrospinal fluid.
Rather than diffusing uniformly through brain tissue, the images revealed fluid moving along pathways — through perivascular spaces in clearly defined channels. Researchers documented the finding with a specific kind of MRI known as fluid attenuated inversion recovery, or FLAIR. This type of imaging is sometimes used following the removal of tumors in the brain. As it turns out, it also revealed the gadolinium tracer in the brain, whereas the standard MRI sequences did not.
“That was the key,” Piantino said.
“You can actually see dark perivascular spaces in the brain turn bright,” said co-lead author Erin Yamamoto, M.D., a resident in neurological surgery in the OHSU School of Medicine. “It was quite similar to the imaging the Rochester group showed in mice.”
Clearing Waste From the Brain
Scientists believe this network of pathways effectively flushes the brain of metabolic wastes generated by its energy-intensive work. Wastes include proteins such as amyloid and tau, which have been shown to form clumps and tangles in brain images of patients with Alzheimer’s disease.
Emerging research suggests medications that may be useful, but much of the focus around the glymphatic system has revolved around lifestyle-based measures to improve the quality of sleep, such as maintaining a regular sleep schedule, establishing a relaxing routine, and avoiding screens in the bedroom before bed. Especially at night during deep sleep, researchers believe a well-functioning glymphatic system efficiently carries waste proteins toward veins exiting the brain.
“People thought these perivascular spaces were important, but it had never been proven,” Piantino said. “Now it has.”
Reference: “The perivascular space is a conduit for cerebrospinal fluid flow in humans: a proof-of-principle report” 7 October 2024, Proceedings of the National Academy of Sciences.
DOI: 10.1073/pnas.2407246121
The authors credited the late Justin Cetas, M.D., Ph.D., who initiated the study as an OHSU neurosurgeon before leaving OHSU to become chair of neurological surgery at his alma mater, the University of Arizona Health Sciences Center in Tucson. He died in a motorcycle accident in 2022.
In addition to Piantino and Yamamoto, authors included co-lead author Jacob H. Bagley, M.D., of OHSU and Aurora St. Luke’s Medical Center in Milwaukee; and co-authors Mathew Geltzeiler, M.D., associate professor of otolaryngology (head and neck surgery) in the OHSU School of Medicine; Olabisi R. Sanusi, M.D., assistant professor of neurological surgery in the OHSU School of Medicine; Aclan Dogan, M.D., professor of neurological surgery (skull base and cerebrovascular) in the OHSU School of Medicine; and Jesse J. Liu, M.D., assistant professor of neurological surgery (skull base and cerebrovascular) in the OHSU School of Medicine.
The research was supported by a Medical Research Foundation of Oregon Early Clinical Investigator grant; the North American Skull Base Society; and by the National Heart, Lung and Blood Institute of the National Institutes of Health, grant awards K23HL150217 and R21HL167077. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.