Plastics underpin much of modern life—areas like medicine, technology, and food safety would be unrecognizable without plastics and their useful properties. However, the toughness of plastics, which is often desirable, also makes them a dangerous pollutant and difficult to recycle. The solution to this serious and growing problem is making plastics easier to recycle.
In a study published in Chemical Science, researchers at Osaka University have found a way to make tough, high-performance polymers, the main component of plastics, that can be broken down easily and precisely into their component parts and recycled into materials that are like new.
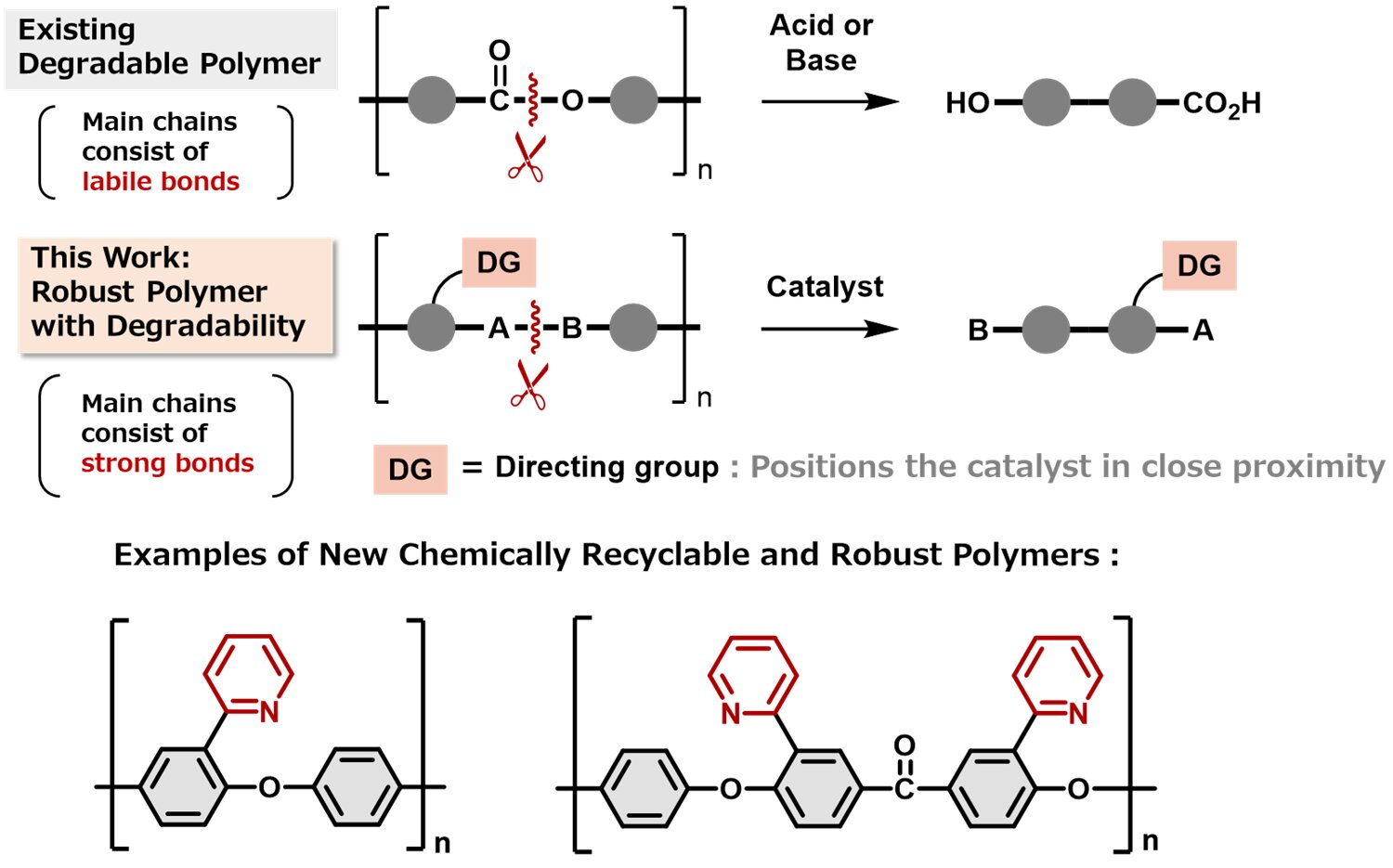
The main component of plastics are molecules called polymers, which are long chains of small repeating units called monomers. Current physical recycling simply reuses the polymers without breaking them down, and the recycled plastic is usually worse than the original.
Chemical recycling is a newer method that breaks the polymer chains back down into their monomer units and then strings the units back together. The recycled plastic is as good as new. However, the polymers designed for chemical recycling are usually weak because they have weak links between the monomer units so that it is easy to break the chains up.
The researchers have developed a way to make tough, chemically recyclable polymers without compromising on heat and chemical resistance. This breakthrough could hugely expand the use of chemically recyclable polymers.
“We knew that we needed to make the links between the monomers really strong in harsh environments but easily broken under specific conditions for recycling,” says lead author Satoshi Ogawa. “We were surprised to find that no one had tried including a directing group, which would break the strong links only in the presence of a metal catalyst.”
The directing group is like a lock on the link, only opening the link when the right key is present. The polymers stood up to high temperatures and harsh chemicals, but when it came to recycling, a nickel catalyst acted like a key, and the directing group opened the links easily, releasing the monomers. The original polymer could then be reassembled from the monomers.
“It’s a huge step forward to make a polymer this tough that can be broken down easily and precisely and recycled into a pristine material in so few steps,” explains senior author Mamoru Tobisu. “This revolutionary design could be used in making high-performance polymers that can be recycled indefinitely with no loss of quality.”
The team’s work shows that there doesn’t have to be a tradeoff between performance and recyclability. Their design could be used in lots of other polymers to make many types of plastic chemically recyclable, potentially helping to consign plastic pollution to the trash can of history.
More information:
Controlled Degradation of Chemically Stable Poly(aryl ethers) via Directing Group-Assisted Catalysis, Chemical Science (2024). DOI: 10.1039/d4sc04147j
Provided by
Osaka University
Citation:
New polymer design breaks the tradeoff between toughness and recyclability (2024, October 7)
retrieved 7 October 2024
from https://phys.org/news/2024-10-polymer-tradeoff-toughness-recyclability.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.