New research reveals that liquid crystals can form dynamic structures that mimic biological transport systems, suggesting potential applications in creating self-assembling materials and modeling biological systems.
Liquid crystals are everywhere. They are used in numerous applications, such as cell phone screens, video game consoles, car dashboards, and medical devices. Due to the unique properties of these fluids, if you run an electric current through liquid crystal displays (LCDs), they generate colors: rearrange their shape, and they will reflect different wavelengths of light.
New Discoveries in Liquid Crystal Structures
Now, researchers at the lab of Chinedum Osuji, Eduardo D. Glandt Presidential Professor and Chair of Chemical and Biomolecular Engineering, have discovered these remarkable crystals may be able to do even more. Under the right conditions, liquid crystals condense into astonishing structures, spontaneously generating filaments and flattened discs that can transport material from one place to another, much like complex biological systems. This insight may lead to self-assembling materials, new ways to model cellular activity, and more.
“It’s like a network of conveyor belts,” says Christopher Browne, a postdoctoral researcher in Osuji’s lab and the co-first author of a recent paper in Proceedings of the National Academy of Sciences (PNAS) that describes the finding. “It was this serendipitous observation of something that superficially looks very lifelike — that was the initial cue that this might be something more general and more interesting.”
Collaborative Research on Condensate Formation
Browne and Osuji are now part of an NSF-supported interdisciplinary group at the Laboratory for Research on the Structure of Matter (LRSM) led by Matthew Good, Associate Professor of Cell and Developmental Biology within the Perelman School of Medicine, and Elizabeth Rhoades, Professor of Chemistry within the School of Arts & Sciences, that is studying condensate formation in biological and non-biological systems.
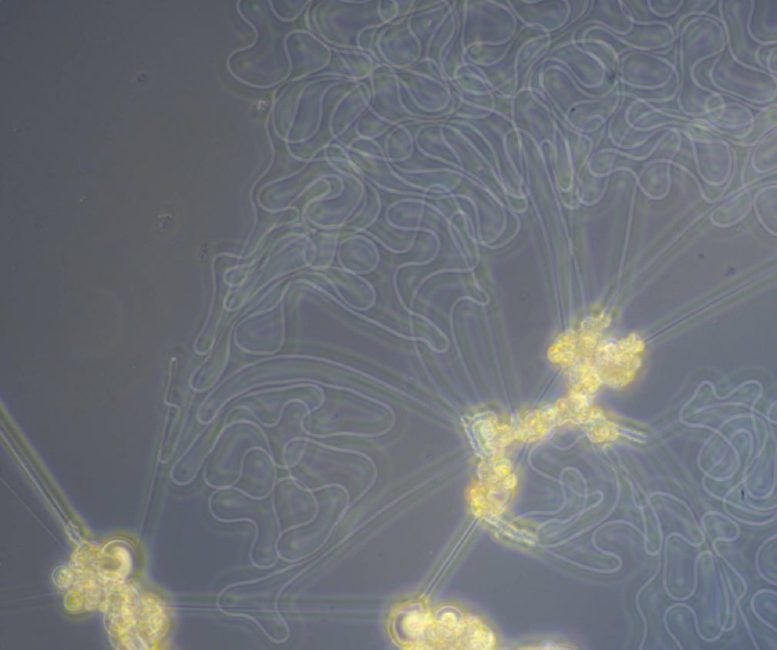
Unusual Behavior in Liquid Crystal Phase Separation
Originally, Osuji’s lab partnered with ExxonMobil to investigate mesophase pitch, a substance used in the development of high-strength carbon fibers, like those found in Formula 1 cars and high-end tennis rackets. “Those materials are liquid crystals,” says Osuji, of the chemical precursors to the carbon fibers themselves. “Or better stated, they are liquid crystalline over some period of their existence during processing.” While experimenting with condensates at different temperatures, Yuma Morimitsu, another postdoctoral fellow in the Osuji Lab and the paper’s other co-first author, noticed unusual behavior in the material.
Normally, if you combine two immiscible — that is, not mixable — fluids and then heat them to a high enough temperature to force them to mix, if you then cool the mixture, at some point, it will separate or “demix.” Typically, this happens by the formation of droplets that coalesce to form a separate layer, much like how, if you combine oil and water, you eventually wind up with a layer of oil on top of the water.
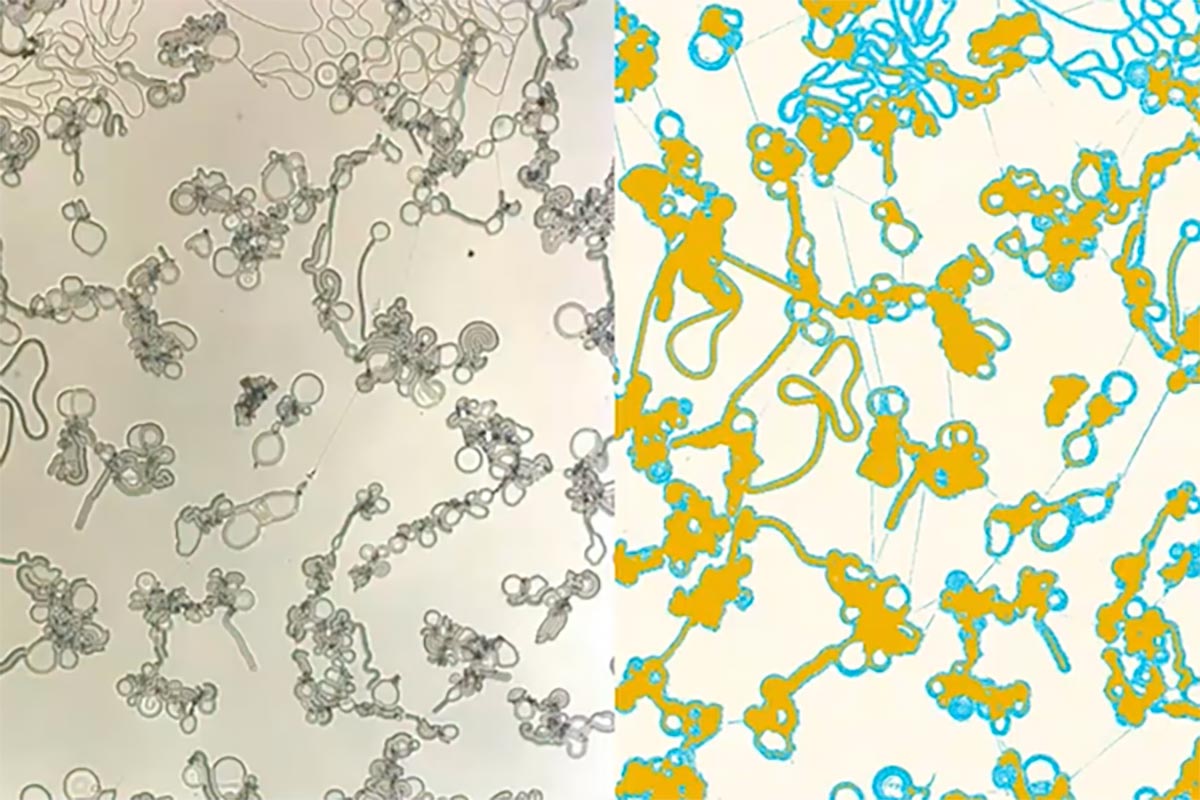
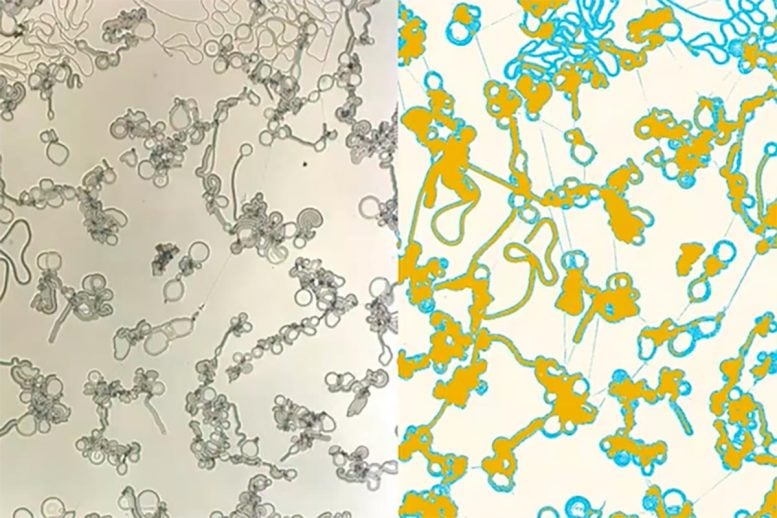
Video showing the liquid crystal condensates forming — the right-hand side uses false color to differentiate the filaments (light blue) and flattened discs (yellow). Video is at 60x real-time and 5x magnification. Credit: Christopher Browne, Chinedum Osuji
Unique Phase Separation and Structural Formation
In this case, the liquid crystal, 4’-cyano 4-dodecyloxybiphenyl, also known as 12OCB, spontaneously formed highly irregular structures when separating from squalane, a colorless oil. “Instead of forming drops,” says Osuji, “when you have this phase separation between the liquid crystal and the other components of the system, you form cascaded structures, the first of which is these filaments, which grow rapidly and thereafter form another set of structures — what we call bulged discs or flat droplets.”
Observations and Implications of Liquid Crystal Behavior
To understand the system, the researchers used powerful microscopes to observe the liquid crystals’ movement on the micrometer scale — that is, millionths of a meter, comparable to the width of a human hair. “The first time we saw these structures, we looked at them at a cooling rate that was excessively high,” recalls Osuji, leading the liquid crystals to clump together. Only by lowering the cooling rate and further zooming in did the researchers realize that the liquid crystals were spontaneously forming structures reminiscent of biological systems.
Interestingly, Browne found, that several researchers had come close to observing similar behavior decades ago, but either studied systems in which the behavior was not particularly pronounced or lacked microscopy powerful enough to visualize what was happening.
Potential Applications and Future Research
For Browne, the result’s most exciting implication is that it brings together several traditionally disparate fields: the world of active matter research, which focuses on biological systems that transport material and produce motion, and the realms of self-assembly and phase behavior, which study materials that create new structures on their own and that behave differently when changing phase. “This is a new type of active matter system,” says Browne.
He and Osuji also point to the possibility of leveraging the findings to emulate biological systems, either to better understand how they work or to manufacture materials. “Molecules are being absorbed into the filaments and then shuttled into those flat droplets continuously,” says Osuji, “even though just by looking at the system, you can’t discern any obvious activity.” In effect, the flat droplets could function like small reactors, churning out molecules that the filaments carry to other droplets for storage or further chemical activity.
The researchers also suggest that their findings could reinvigorate research into liquid crystals themselves. “When a field becomes industrialized,” says Browne, “oftentimes the fundamental research tapers off. But sometimes there are lingering puzzles that nobody finished solving.”
Reference: “Spontaneous assembly of condensate networks during the demixing of structured fluids” by Yuma Morimitsu, Christopher A. Browne, Zhe Liu, Paul G. Severino, Manesh Gopinadhan, Eric B. Sirota, Ozcan Altintas, Kazem V. Edmond and Chinedum O. Osuji, 13 September 2024, Proceedings of the National Academy of Sciences.
DOI: 10.1073/pnas.2407914121
This study was conducted at the University of Pennsylvania, in the School of Engineering and Applied Science’s Department of Chemical and Biomolecular Engineering and the School of Arts & Sciences’ Department of Physics and Astronomy, and ExxonMobil’s Research Division. The work was supported by a grant from ExxonMobil and by the U.S. National Science Foundation (DMR-2309043).