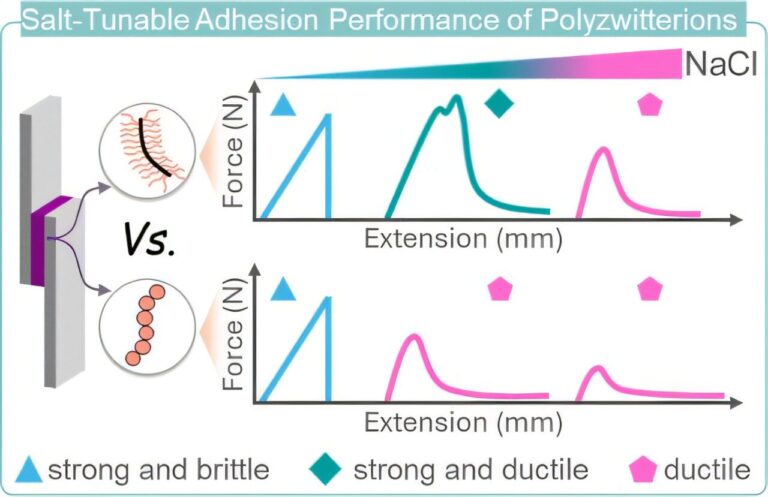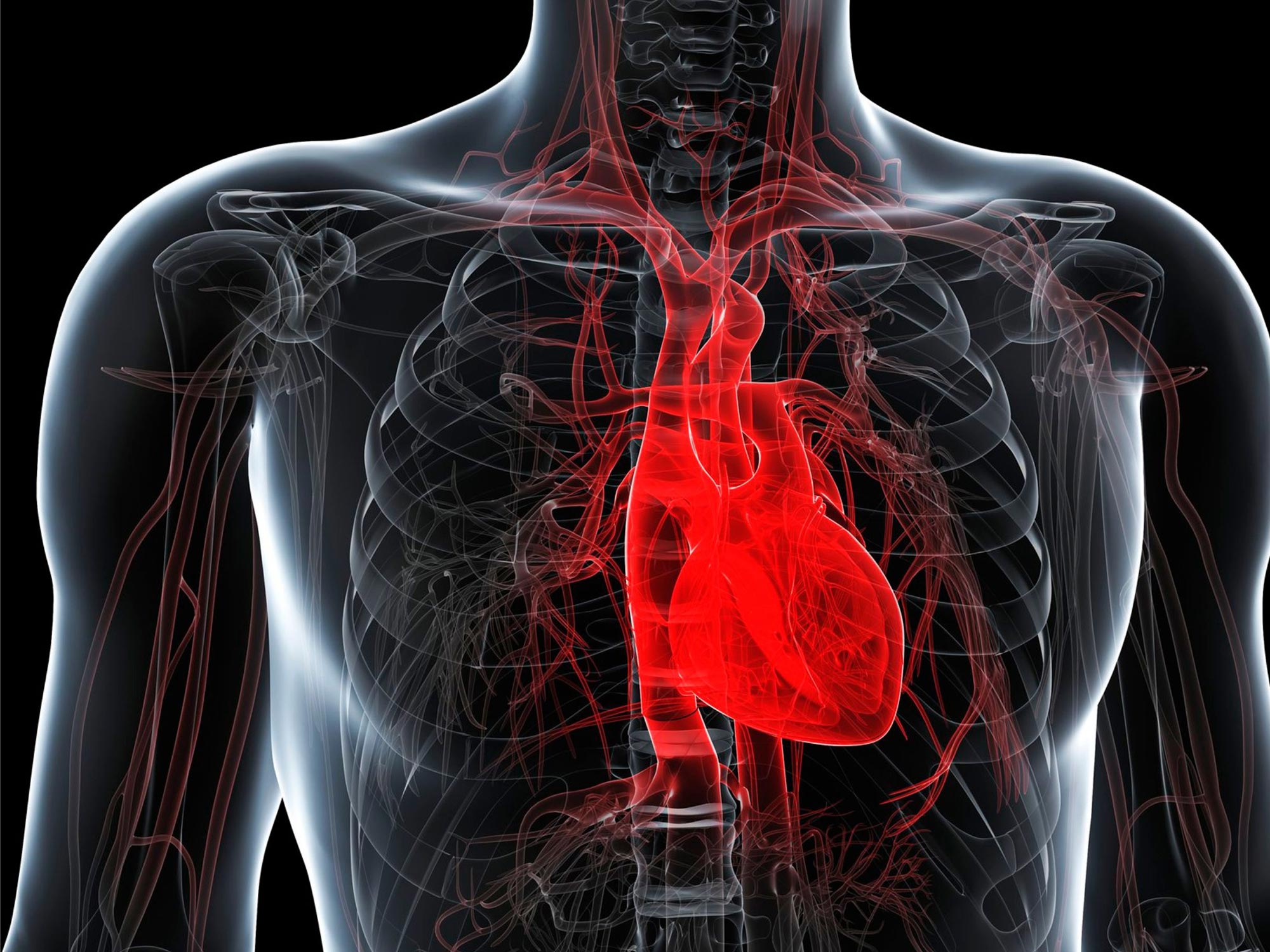
Researchers at the Max Delbrück Center have uncovered genetic distinctions between human hearts and those of other primates. This study highlights evolutionary changes specific to humans and offers fresh perspectives on heart disease.
Researchers from the Hübner and Diecke Labs at the Max Delbrück Center have uncovered genetic differences between human and non-human primate hearts. Published in Nature Cardiovascular Research, their study highlights evolutionary changes in human hearts and offers fresh perspectives on heart disease.
Humans are 98-99% genetically similar to chimpanzees. What then accounts for our differences? Over the years, researchers have shown that the regulation of gene expression – when, where, and by how much genes are switched on – is in large part responsible for our divergent evolutionary trajectories.
Now, researchers in the Cardiovascular and Metabolic Sciences Lab of Professor Norbert Hübner and the Pluripotent Stem Cells Platform of Dr. Sebastian Diecke at the Max Delbrück Center have unveiled surprising differences in gene expression in the hearts of humans and non-human primates. The research, led by Dr. Jorge Ruiz-Orera and published in the journal Nature Cardiovascular Research, points to adaptations in the way genes are regulated that distinguish our hearts from those of our closest evolutionary relatives. It also serves as a warning against extrapolating research from animal hearts to human hearts.
“One of the most surprising findings was how gene regulation in the human heart differs so much from other primates,” says Ruiz-Orera. In terms of anatomy, most mammalian hearts are similar. “But we have many unique evolutionary innovations in terms of gene regulation or translation of proteins,” he adds.
The researchers found hundreds of genes and microproteins – tiny proteins that have been previously identified in human organs but whose function has mostly been a mystery – present in human hearts but not in the hearts of other primates, rats, or mice. “Many of these human genes and microproteins are also abnormally expressed in heart failure, which suggests they could play important roles in cardiac function and disease and may present new targets for therapy,” says Ruiz-Orera.
Comparing gene transcription and translation
The team analyzed heart tissue from chimpanzees and macaques obtained from the biobank of Dr. Ivanela Kondova in the Biomedical Primate Research Centre in Rijswijk, Netherlands, as well as stored heart tissue from humans, rats, and mice that has been used in the lab’s previous research.
Using RNA sequencing, the researchers first mapped and quantified RNA molecules from heart tissues, which provided a comprehensive view of gene expression across different species. To focus specifically on the RNA regions being translated into proteins, the researchers used Ribo-seq to sequence RNA fragments that were actively being translated in each cell. This gave insight into which genes produce functional proteins. By integrating data from these technologies, the team created the most comprehensive resource to date on gene and protein activity in human and non-human primate hearts.
In addition, the researchers used induced pluripotent stem cell-derived cardiomyocyte (iPSC-CM) cell cultures as a model to study how genes are expressed during heart development in humans and other primates. iPSC-CMs are a useful model because they can be grown from adult primate skin cells that have been reprogrammed to an embryonic-like state. These cells turn into cardiomyocytes, the basic cell unit of the heart, enabling researchers to study them during different stages of development.
The discovery that specific microproteins – which are encoded in the genome by snippets of DNA called small open reading frames (ORFs) – are uniquely expressed or translated in human heart cells at different developmental stages suggests that some of these genetic elements may have evolved specifically to meet the demands of the human heart, says Ruiz-Orera. (ORFs lack the classic hallmarks of protein-coding genes and are therefore not classified as genes.)
“Our hearts have different energy demands compared to those of smaller primates like macaques, who have much faster heart rates,” he explains. “This difference seems to be reflected in the regulation of genes related to energy production in the heart. These evolutionary adaptations may also be linked to our bipedalism, lifestyle, and diet.”
In all, the team identified over 1,000 species-specific genomic adaptations, including 551 genes and 504 microprotein coding regions that are only found in human hearts. Among these, they found 76 genes that were common to both humans and other primates and mammals, but only in the human species did they evolve to be expressed in the heart.
Implications for heart disease and use of animals
The researchers showed that some of the genes and microproteins that are specific to humans are dysregulated in conditions like dilated cardiomyopathy, highlighting a potential role in the development of cardiac diseases and suggesting a new target for therapy.
The study also raises important issues about using animals such as mice to study the genetics of human cardiac disease. “Our findings suggest that the differences between species could sometimes result in misleading results,” says Ruiz-Orera. “There are many genes expressed in the human heart that simply aren’t expressed in the hearts of other species.”
In humans, for example, the gene SGLT1 is expressed in the heart. But in non-human primates, rats, and mice, it is expressed only in the kidneys. Dual inhibitors of SGLT1 and SGLT2 have been shown to reduce heart failure, even though their exact role in the heart remains a mystery, says Ruiz-Orera. But since it isn’t expressed in the hearts of other animals, researchers will not be able to learn much by testing such therapies in these models. “It highlights the importance of considering evolutionary context in medical research,” he adds.
Reference: “Evolution of translational control and the emergence of genes and open reading frames in human and non-human primate hearts” by Jorge Ruiz-Orera, Duncan C. Miller, Johannes Greiner, Carolin Genehr, Aliki Grammatikaki, Susanne Blachut, Jeanne Mbebi, Giannino Patone, Anna Myronova, Eleonora Adami, Nikita Dewani, Ning Liang, Oliver Hummel, Michael B. Muecke, Thomas B. Hildebrandt, Guido Fritsch, Lisa Schrade, Wolfram H. Zimmermann, Ivanela Kondova, Sebastian Diecke, Sebastiaan van Heesch and Norbert Hübner, 24 September 2024, Nature Cardiovascular Research.
DOI: 10.1038/s44161-024-00544-7