Plant molecular farming (PMF) is a modern, sophisticated technology that utilizes plants’ biosynthetic machinery to synthesize a plethora of recombinant proteins, including industrial and therapeutic enzymes. It has several advantages over traditional approaches, such as microbial fermentation and mammalian cell culture, including lower costs, high-yield production, and absence of human pathogens and endotoxins. Plants also provide considerable flexibility that allows customized protein production.
Tobacco species like Nicotiana benthamiana and Nicotiana tabacum are known for having compromised basal immunity and a less robust ribonucleic acid (RNA) silencing pathway involving the degradation of foreign RNA as a defense mechanism. This, along with its short life cycle and large biomass production capacity, makes it an ideal choice for quick and efficient recombinant protein production.
However, despite these multiple advantages, there are certain limitations to this protein production. Tobacco cells require engineering for specific subcellular localization of each recombinant protein.
While multiple studies explored the recombinant protein production in tobacco, a comprehensive study on subcellular localization strategies was required. A review published in BioDesign Research addresses this issue and focuses on targeting strategies for directing the recombinant proteins to four subcellular compartments—endoplasmic reticulum (ER), vacuole, chloroplast and apoplast.
Providing more context, Dr. Shi-Jian Song, a researcher from the Chinese Academy of Agricultural Sciences, China and one of the corresponding authors of this study, says, “Optimizing subcellular localization for individual target proteins is crucial for successful protein synthesis and its utilization in the pharmacological industry.”
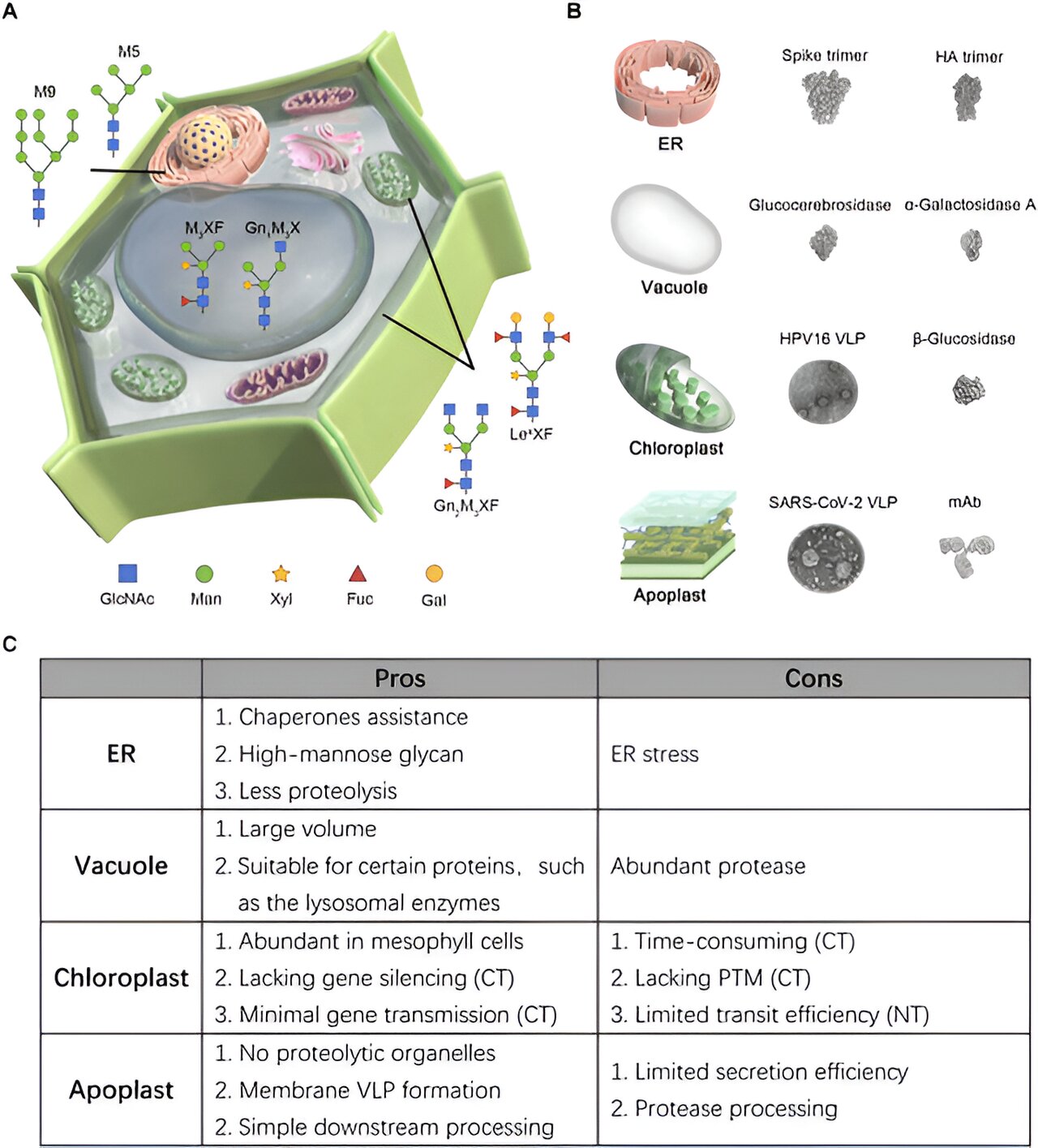
ER is recognized for recombinant protein localization as it houses molecular chaperons that aid protein-folding and also minimizes risks of protein degradation. Proteins directed towards the ER undergo homogenous glycosylation, a reaction involving the addition of carbohydrates, which is essential for many therapeutic proteins. The accumulation of recombinant proteins in the plant ER can be achieved by incorporating an N-terminal specific ER targeting or secretion signal peptide along with a C-terminal retention sequence.
While highlighting the limitations of the study, Dr. Inhwan Hwang, the other corresponding author of this study, mentions, “Overloading the ER might cause ER stress resulting in a significantly reduced protein yield in such instances.” This can be avoided if careful management is done during expression levels.
The findings of this study suggest ER localization is optimal for large, complex glycoproteins (proteins with carbohydrates attached) that need chaperons for folding. These glycoproteins have a pattern of carbohydrates attached to the nitrogen atom, similar to mammalian proteins.
Plant vacuole is another crucial organelle in tobacco, occupying about 80% to 90% of cell volume in tobacco leaves. The PMF technology utilizes this huge storage capacity for recombinant protein localization. Vacuolar sorting signals, that can be either location specific or sequence specific, are critical for vacuolar targeting.
Pointing out an interesting aspect about these vacuoles, Dr. Hai-Ping Diao says, “The proteins can enter the vacuole via different trafficking routes. recombination is done to ensure that the protein is directly transported from the ER to the vacuole, bypassing the Golgi apparatus.”
Certain proteins also tend to degrade in the vacuole due to the presence of a breakdown enzyme called protease. So, it is best to localize the proteins that are tolerant to acidic environments or are naturally localized in the human lysosome.
Chloroplasts in tobacco leaf tissues store the highest level of native proteins, making it ideal for accumulating large quantities of recombinant protein. There are two primary strategies for accumulating large quantities of recombinant proteins—chloroplast transformation and nuclear transformation.
Chloroplast transformation allows stable expression of foreign genes, optimal protein-folding conditions, and minimal risk of environmental transfer. However, generating high-yielding transgenic plants through this process is difficult and time-consuming due to certain technical challenges.
Nuclear transformation, on the other hand, involves a recombinant protein fused to a chloroplast transit peptide, allowing faster protein production. The study indicates that chloroplast-localization may work best for proteins that do not require extensive biochemical modifications.
Plant apoplast, a crucial space between cell-membrane and cell-wall in plant cells, is considered as an excellent site for recombinant protein accumulation. The protein accumulation in the apoplast also simplifies the purification method.
While the smaller recombinant proteins can be directly extracted from the apoplast fluid, large protein complexes require a conventional purification process. Recombinant proteins may also have compromised structural integrity due to the presence of protease in the apoplast. To evade this complication, co-expression of a protease inhibitor is increasingly being utilized as an emerging strategy.
PMF has the potential to revolutionize recombinant protein production. However, adaptable production levels, comparable qualities, and cost-issues remain. Elaborating further, Dr. Shi-Jian Song says, “To normalize the use of transgenic plants in industrial research, it is important to strictly adhere to protocols, improve public engagement, and follow robust safety protocols.”
Remodeling the tobacco plant chassis, including low-efficiency protease processing, allocating resources effectively, and establishing a toxin-free plant reactor can help in further advancements. The eventual commercialization of biomanufacturing is a crucial sign of PMF development.
More information:
Shi-Jian Song et al, Advances in Subcellular Accumulation Design for Recombinant Protein Production in Tobacco, BioDesign Research (2024). DOI: 10.34133/bdr.0047
Provided by
NanJing Agricultural University
Citation:
Strategies for maximizing recombinant protein production in tobacco plants (2024, September 25)
retrieved 25 September 2024
from https://phys.org/news/2024-09-strategies-maximizing-recombinant-protein-production.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.