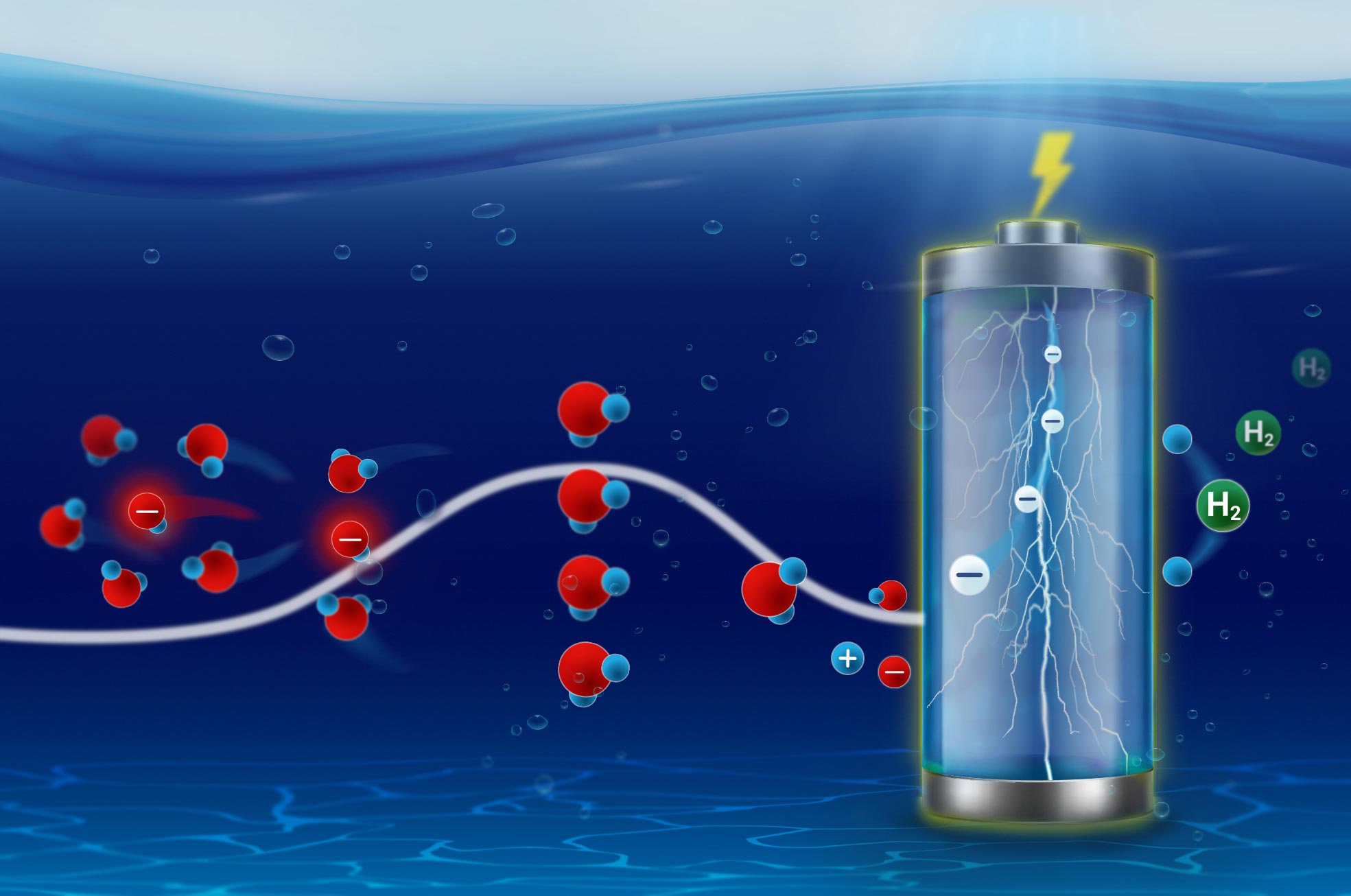
Recent research at the Interface Science Department of the Fritz- Haber Institute has shed light on the electrosorption kinetics of ions and their reorganization at electrocatalyst surfaces.
The study, which leverages transition state theory and modern spectroscopic techniques, reveals critical insights into how activation entropy and structural surface features like defects impact catalytic reactions. This deep understanding may lead to the development of more effective catalysts for energy conversion and chemical production.
Ion’s Pathway and Solvation Dynamics
The ion’s pathway is strongly influenced by a process that is ubiquitous across bio- and electrochemistry: ions need to reorganize their solvation shell before they can intercalate into battery cathodes, enter ion channels across biochemical membranes or adsorb, and convert to chemicals, such as green hydrogen, on electrocatalyst surfaces.
Previously, the team discovered that the kinetics of interfacial ion solvation are governed by so-called compensation effects between the activation entropy and enthalpy. In other words, as the elevation of the mountain in front of the ion is raised, the number of available hiking trails increases, making it more likely for the ion to go on the hike. To come to these conclusions the team interpreted the kinetics according to statistical physics and the Eyring-Evans-Polanyi equation, the centerpiece of transition state theory from 1935, which was co-developed by Michael Polanyi, head of the Physical Chemistry Department of FHI until 1933.
Advances in Transition State Theory
Now, almost 90 years later, the researchers of the Interface Science Department are able to tract the two key parameters of transition state theory, the activation enthalpy and activation entropy with a millisecond time resolution.
“Our findings are really substantial on many fundamental levels,” says Francisco Sarabia, first author of the study and Marie Curie Postdoctoral Fellow. “Using this technique, we can directly access the electrosorption kinetics of hydroxide ions that occur at specific structural surface motifs, e.g., step-edges or defects, and show how they are linked to electrocatalyst kinetics. Further, we studied the dynamic poisoning behavior of the Pt surface during the ammonia oxidation reaction and how it impacts the solvation kinetics. This level of insight has remained completely hidden, so far.”
Overall, the work further supports the notion that activation entropy changes at the catalyst surface and in the interfacial solvent are critical to understanding electrocatalyst activity. For example, the team discovered that the pH can directly impact the activation entropy and induce non-Nernstian activity changes with pH. Currently, it is widely assumed, that the activation energy plays the primary role in the bias dependence of electrocatalytic reactions.
Real-Time Insights Into Electrocatalyst Activity
Dr. Sebastian Öner, Group leader at the Interface Science Department and corresponding author of the study, further emphasizes the importance of these findings. “Abundant operando spectroscopy and microscopy evidence, including from my colleagues here at the Inorganic Chemistry and Interface Science Departments, show that catalyst surfaces are highly dynamic. Beyond studying solvation kinetics, we now have a tool, that we can apply to capture true kinetic information in real-time and overlay it with spectroscopic and microscopic information.”
The research of the team highlights the importance of bias dependent changes in the local environment of catalysts, showing how the solid structure and liquid electrolyte are closely interconnected and can influence each other. This comprehensive understanding is crucial for developing catalysts with improved activity, selectivity, and stability.
Impact on Energy and Chemical Conversion
The Interface Science Department, led by Prof. Dr. Beatriz Roldán Cuenya, is committed to further exploring these insights, with the potential to significantly impact the fields of energy and chemical conversion technology.
Reference: “Exploring dynamic solvation kinetics at electrocatalyst surfaces” by Francisco Sarabia, Carlos Gomez Rodellar, Beatriz Roldan Cuenya and Sebastian Z. Oener, 18 September 2024, Nature Communications.
DOI: 10.1038/s41467-024-52499-9