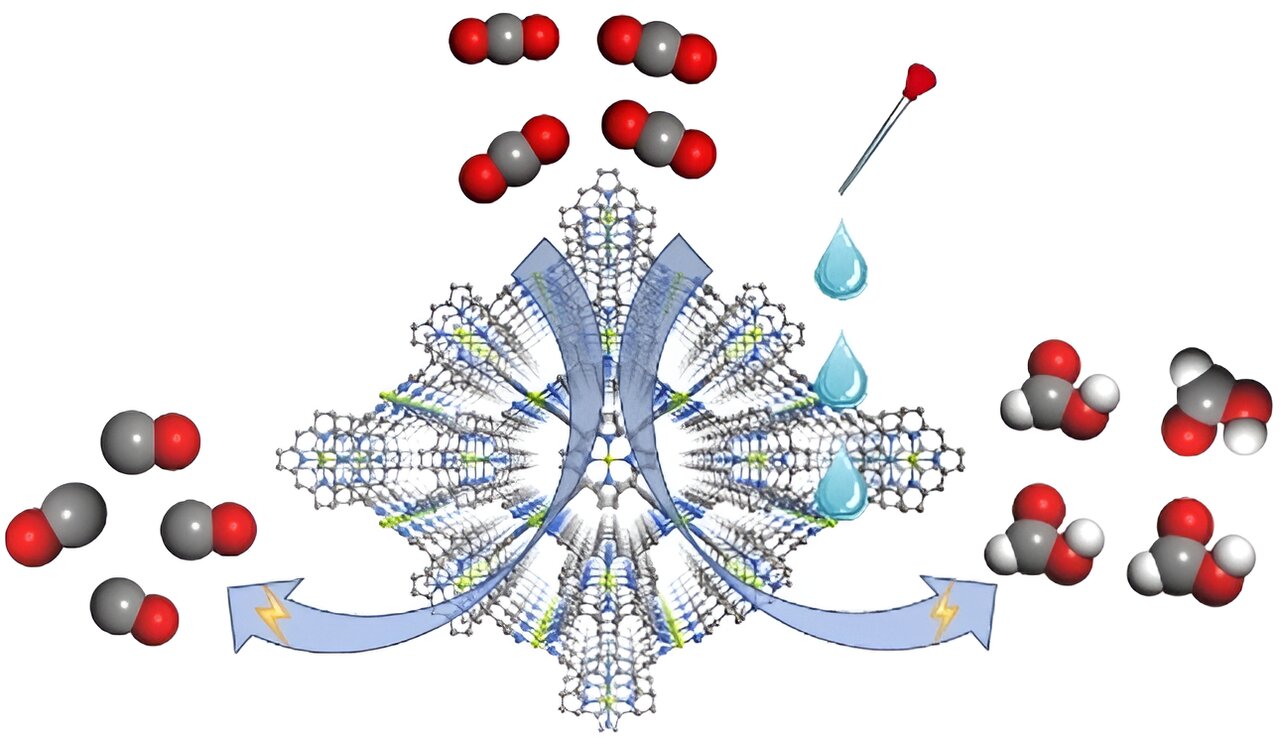
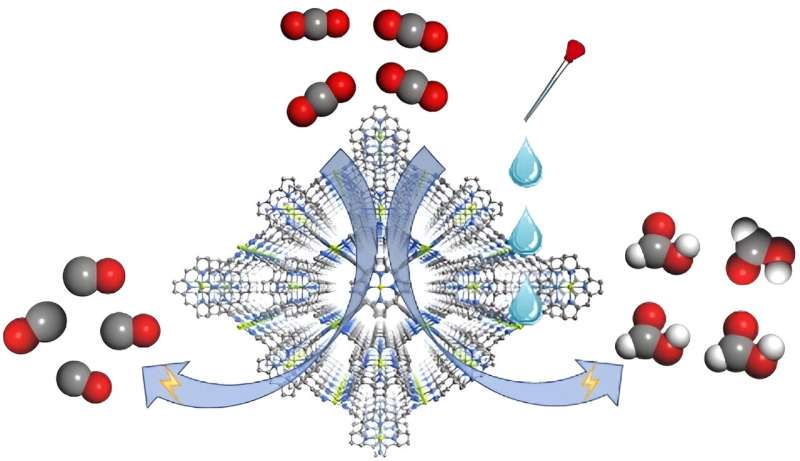
Electrochemical reduction of CO2 has long been viewed as a promising approach to utilize CO2. Selective generation is an important topic, but most efforts on controlling product selectivity have been devoted to catalyst design and modification. The impact of electrolyte composition is less studied.
In a study published in Angewandte Chemie International Edition, researchers report a metal-organic framework (MOF) electrocatalyst that exhibits CO2 reduction product selectivity depending on the composition of electrolytes.
This finding provides a possible approach to control and tune reaction selectivity toward value-added CO2 reduction products.
The team includes Prof. Cao Rong, Prof. Zhang Teng, and colleagues from Fujian Institute of Research on the Structure of Matter of the Chinese Academy of Sciences. They constructed the MOF catalyst, namely FICN-8, from Cu(porphyrin)-derived ligands and Cu(pyrazolate) building units.
FICN-8 has a three dimensional porous structure that ensures substrate-accessibility of the metalloporphyrin catalytic sites. It is used as a heterogenous electrocatalyst which allows systematic solvent/electrolyte composition tuning in a wide range.
Electrochemical tests revealed that FICN-8 has a high activity for electrochemical CO2 reduction. In tetrabutylammonium hexafluorophosphate (TBAPF6)/acetonitrile (MeCN) electrolyte, FICN-8 catalyzed the reduction of CO2 to CO with a high selectivity up to 95%.
When water or trifluoroethanol (TFE) was added to the electrolyte as a proton source, the major CO2 reduction product gradually switched from CO to formic acid. The highest observed Faradaic efficiency for formic acid production reached 48% with 2.65 mol/L water or 0.55 mol/L TFE addition.
The researchers also designed a series of experiments to find out the reaction mechanism that accounts for this selectivity switching. Kinetic isotope effect (KIE) measurements gave a near-identity kH/kD KIE value for CO production and a large KIE value of 3.7±0.7 for formic acid production, suggesting direct involvement of protons in the reaction path of HCOOH formation.
Theoretical calculations identified reductive adsorption of hydride (*H) on the porphyrin N site as the crucial step for HCOOH formation, while production of CO follows another route that is independent of proton concentration.
This study highlights the importance of electrolyte composition in product selectivity of electrochemical CO2 reduction, and opens up the possibility of constructing novel catalyst-electrolyte systems for value-added CO2 reduction products.
More information:
Si‐Hua Pu et al, Electrolyte Composition‐Dependent Product Selectivity in CO2 Reduction with a Porphyrinic Metal–Organic Framework Catalyst, Angewandte Chemie International Edition (2024). DOI: 10.1002/anie.202411766
Provided by
Chinese Academy of Sciences
Citation:
Scientists discover electrolyte composition-dependent CO₂ reduction selectivity with an MOF catalyst (2024, September 25)
retrieved 25 September 2024
from https://phys.org/news/2024-09-scientists-electrolyte-composition-reduction-mof.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.