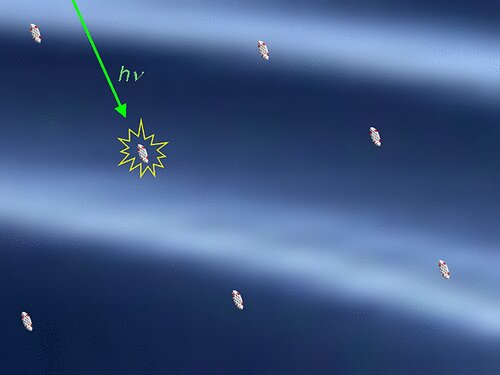
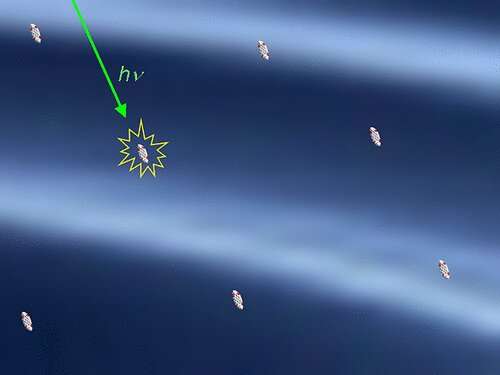
Intermolecular interactions are the forces that pertain between molecules. In general, these interactions scarcely extend beyond the boundaries of molecules. For the most part, they are effective over distances of less than 1 nanometer (10-9 m).
The largest distances discovered to date were in energy transmissions, where almost 10 nanometers were reached. A team led by LMU chemist Heinz Langhals has now found intermolecular interactions which, to the astonishment of the scientists, extend beyond 100 nanometers.
The researchers were able to demonstrate this using the concentration-dependent fluorescence decay time of dyes. “In this way, molecules can not only interact with their neighbors, but do so up to almost macroscopic dimensions,” says Langhals.
In the view of the authors, this raises new possibilities for addressing molecular structures macroscopically, making it an interesting prospect for molecular memories.
The research was published in The Journal of Physical Chemistry Letters.
New study refutes theory on transfer of light energy
Heinz Langhals et al, Dependence of the Fluorescent Lifetime τ on the Concentration at High Dilution, The Journal of Physical Chemistry Letters (2022). DOI: 10.1021/acs.jpclett.2c01447
Ludwig Maximilian University of Munich
Citation:
Chemist shows that intermolecular interactions can attain previously unknown dimensions (2022, August 24)
retrieved 24 August 2022
from https://phys.org/news/2022-08-chemist-intermolecular-interactions-previously-unknown.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.