A new study reveals that azoles, the most widely-used antifungals, cause fungal pathogens to self-destruct by triggering cellular “suicide” programs. This discovery, which details how azoles inhibit ergosterol production leading to cell death through mechanisms like apoptosis and macroautophagy, offers new insights that could help manage resistance and improve strategies for protecting food security and human health. Credit: SciTechDaily.com
New research led by the University of Exeter shows that azole antifungals trigger self-destruction in fungal pathogens by inhibiting ergosterol production, potentially aiding efforts to combat fungal resistance and safeguard food and health.
Scientists have found that the world’s most commonly used class of antifungals causes pathogens to self-destruct. This discovery, led by the University of Exeter, could enhance methods to safeguard food security and protect human health.
Fungal infections result in the loss of up to a quarter of global crop yields. They also present serious risks to human health, potentially proving lethal for individuals with compromised immune systems.
Importance of Azole Fungicides
Our strongest “weapon” against fungal plant diseases is azole fungicides. These chemical products account for a quarter of the world’s agricultural fungicide market, worth more than £3 billion (~$3.8 billion) annually. Antifungal azoles are also widely used as a treatment against pathogenic fungi which can be fatal to humans, which adds to their importance in our attempt to control fungal disease.
Azoles target enzymes in the pathogen cell that produce cholesterol-like molecules, named ergosterol. Ergosterol is an important component of cellular bio-membranes. Azoles deplete ergosterol, which results in the killing of the pathogen cell. However, despite the importance of azoles, scientists know little about the actual cause of pathogen death.
New Insights From the University of Exeter
In a new study published today (May 31) in Nature Communications, University of Exeter scientists have uncovered the cellular mechanism by which azoles kill pathogenic fungi.
Funded by the BBSRC, the team of researchers, led by Professor Gero Steinberg, combined live-cell imaging approaches and molecular genetics to understand why the inhibition of ergosterol synthesis results in cell death in the crop pathogenic fungus Zymoseptoria tritic (Z. tritici). This fungus causes septoria leaf blotch in wheat, a serious disease in temperate climates, estimated to cause more than £250 million per year in costs in the UK alone due to harvest loss and fungicide spraying.
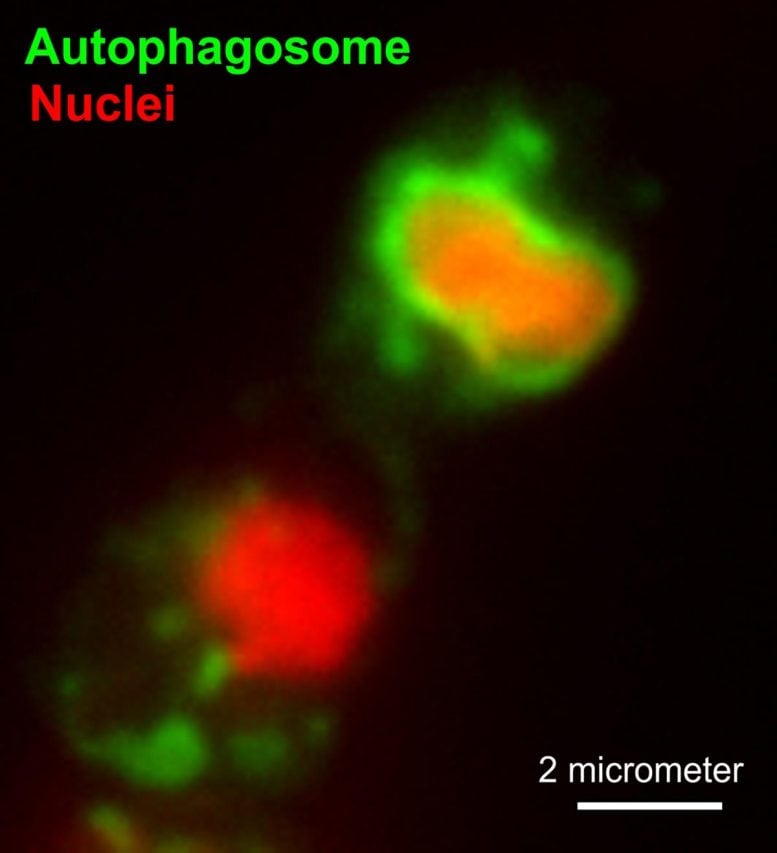
Figure 1: An autophagosome (green) in the process of “eating” a nucleus (red) in a azole-treated cell of Z. tritici. Credit: Dr. Martin Schuster
Mechanisms of Azole-Induced Cell Death
The Exeter team observed living Z. tritici cells, treated them with agricultural azoles and analyzed the cellular response. They showed that the previously accepted idea that azoles kill the pathogen cell by causing perforation of the outer cell membrane does not apply. Instead, they found that azole-induced reduction of ergosterol increase the activity of cellular mitochondria, the “powerhouse” of the cell, required to produce the cellular “fuel” that drives all metabolic processes in the pathogen cell. While producing more “fuel” is not harmful in itself, the process leads to the formation of more toxic by-products. These by-products initiate a “suicide” program in the pathogen cell, named apoptosis. In addition, reduced ergosterol levels also trigger a second “self-destruct” pathway, which causes the cell to “self-eat” its own nuclei and other vital organelles – a process known as macroautophagy (Figure 1). The authors show that both cell death pathways underpin the lethal activity of azoles. They conclude that azoles drive the fungal pathogen into “suicide” by initiating self-destruction.
Broader Implications and Future Directions
The authors found the same mechanism of how azoles kill pathogen cells in rice-blast fungus Magnaporthe oryzae. The disease caused by this fungus kills up to 30 percent of rice, an essential food crop for more than 3.5 billion people across the world. The team also tested other clinically relevant anti-fungal drugs that target the ergosterol biosynthesis, including Terbinafine, Tolfonate, and Fluconazole. All initiated the same responses in the pathogen cell, suggesting that cell suicide is a general consequence of ergosterol biosynthesis inhibitors.
Lead author Professor Gero Steinberg, who holds a Chair in Cell Biology and is Director of the Bioimaging Centre at the University of Exeter, said: “Our findings rewrite common understanding of how azoles kill fungal pathogens. We show that azoles trigger cellular “suicide” programs, which result in the pathogen self-destructing. This cellular reaction occurs after two days of treatment, suggesting that cells reach a “point of no return” after some time of exposure to azoles. Unfortunately, this gives the pathogen time to develop resistance against azoles, which explains why azole resistance is advancing in fungal pathogens, meaning they are more likely to fail to kill the disease in crops and humans.
“Our work sheds light on the activity of our most widely used chemical control agents in crop and human pathogens across the world. We hope that our results prove to be useful to optimise control strategies that could save lives and secure food security for the future.”
Reference: “Azoles activate type I and type II programmed cell death pathways in crop pathogenic fungi” 31 May 2024, Nature Communications.
DOI: 10.1038/s41467-024-48157-9
Co-authors are Dr. Martin Schuster and Dr. Sreedhar Kilaru at the University of Exeter.