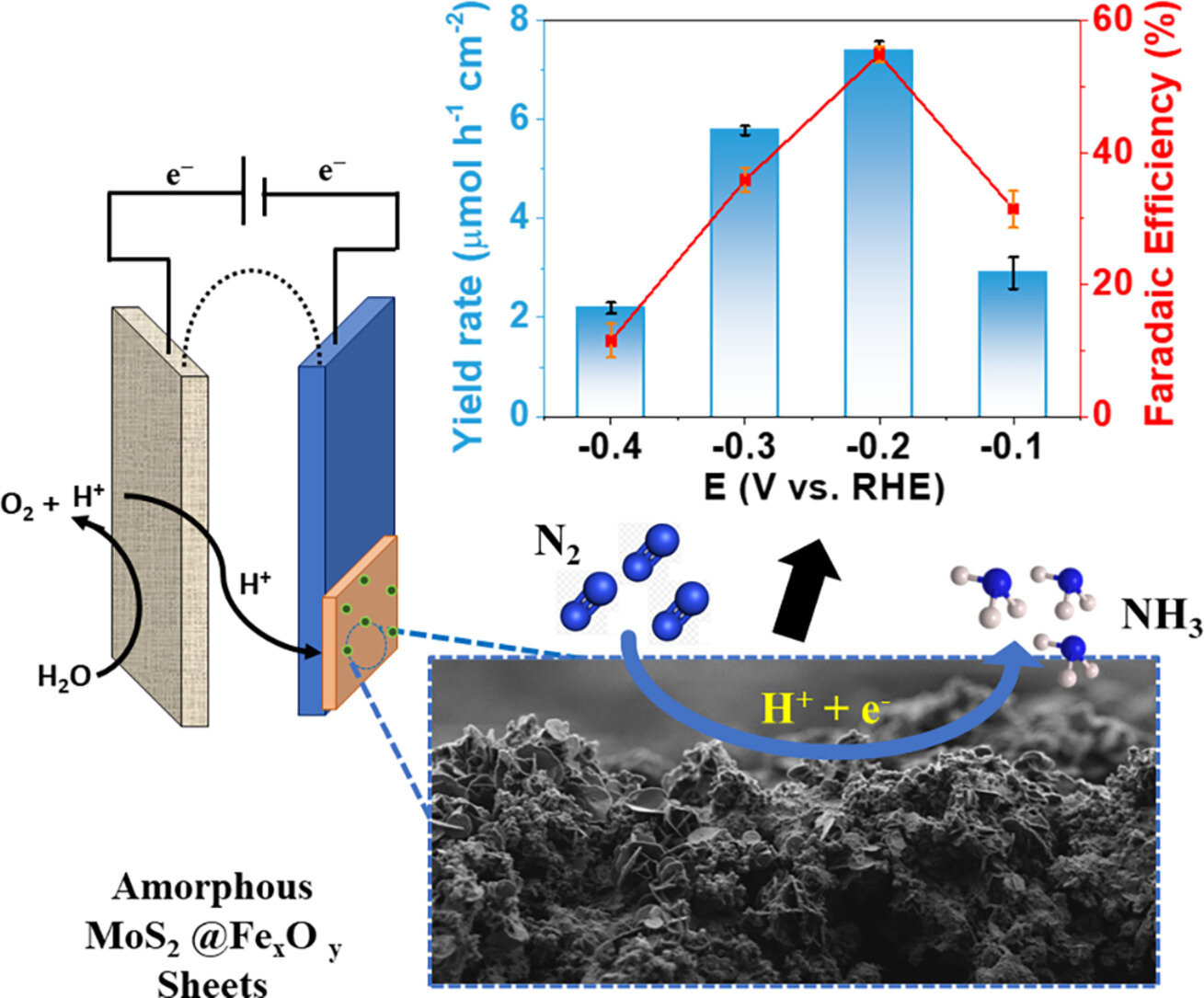
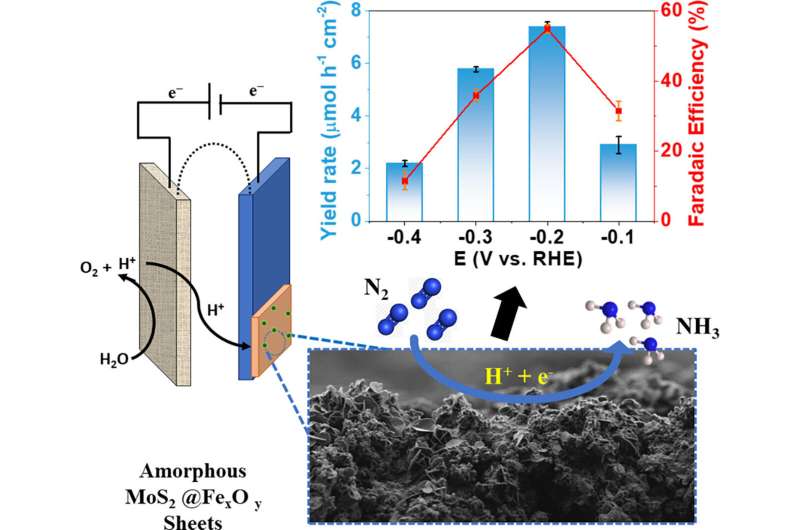
Ammonia is one of the most widely produced chemicals in the world, and is used in a great many manufacturing and service industries. The conventional production technology is the Haber-Bosch process, which combines nitrogen gas (N2) and hydrogen gas (H2) in a reactor in the presence of a catalyst.
This process requires high levels of temperature and pressure, resulting in substantial power consumption. Indeed, ammonia production is estimated to consume 1%–2% of the world’s electricity and to account for about 3% of global carbon emissions.
In pursuit of more sustainable alternatives, researchers affiliated with the Center for Development of Functional Materials (CDMF) have developed an electrochemical nitrogen reduction process using catalysts made of iron oxide and molybdenum disulfide. Because the process is electrochemical, it does not require high temperature and pressure.
An article on the subject is published in the journal Electrochimica Acta. The authors are Caio Vinícius da Silva Almeida, a postdoctoral fellow at UFSCar, and Lucia Helena Mascaro, a professor in UFSCar’s Department of Chemistry.
The catalysts in question are prepared by electrodeposition, a simple and inexpensive method. As reported in the article, they are efficient, stable and durable. The results of the research open up possibilities for the use of simple, low-cost catalysts in ammonia production and the synthesis of amorphous materials for nitrogen fixation.
More information:
Caio V.S. Almeida et al, Enhancing electrochemical N2 reduction at mild conditions with FexOy co-deposited on amorphous MoS2, Electrochimica Acta (2023). DOI: 10.1016/j.electacta.2023.143680
Citation:
Scientists develop catalyst designed to make ammonia production more sustainable (2024, March 21)
retrieved 21 March 2024
from https://phys.org/news/2024-03-scientists-catalyst-ammonia-production-sustainable.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.

