Alkenes represent one of the most abundant classes of organic molecules, which are available in bulk quantities from petroleum and renewable resources, with various utilization in agrochemistry, pharmacy, and organic materials. Typically, the transformation of alkenes by cleavage of both the σ bond and π bond underpins a range of industrial processes and provides methods for the reconstruction of hydrocarbon framework.
Oxidative cleavage is undoubtedly the most common type of scissions of alkene C=C bonds, providing a practical synthetic route to oxygen-enriched compounds. Besides, alkene metathesis represents an alternative method that involves cleavage of alkene C=C bonds and their reformation, resulting in a statistical redistribution of alkylidene fragments under redox-neutral conditions.
While oxidative C=C bond cleavage and alkene metathesis have been extensively investigated, reductive C=C bond cleavage, which might provide a huge opportunity for reactions with different electrophiles to generate valuable compounds with diverse functionality, has rarely been investigated.
Carbon dioxide (CO2) is an ideal C1 synthon for the synthesis of a variety of bulk and fine chemicals because of its abundance, affordability, accessibility, non-toxic nature, and recyclability. However, due to its thermodynamic stability and kinetic inertia, CO2 is challenging to convert efficiently and selectively under mild conditions. Therefore, it is an important and challenging research direction to efficiently utilize CO2 under mild conditions.
With the increasing focus on green synthesis and environmental concerns, photochemistry has been acknowledged as a potent technique for a wide range of organic transformations. Recently, the radical pathway for C=C bond cleavage has garnered increasing attention and has enabled many transformations that complement traditional ionic processes. Furthermore, there have been numerous reported cases of photocatalytic CO2 activation reactions.
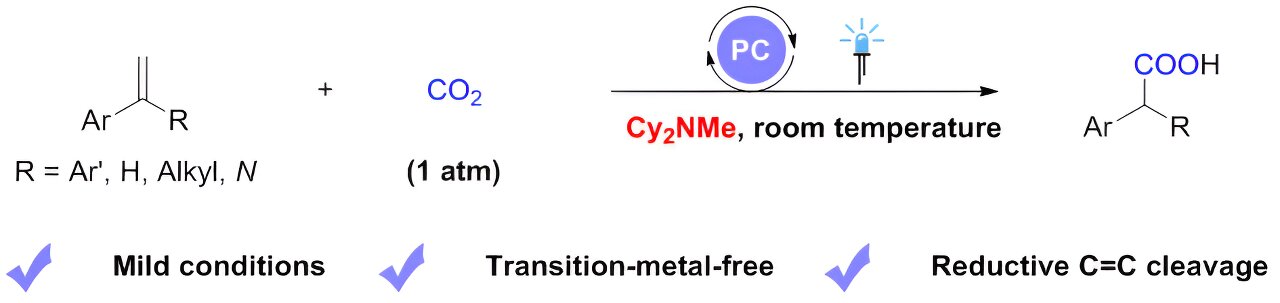
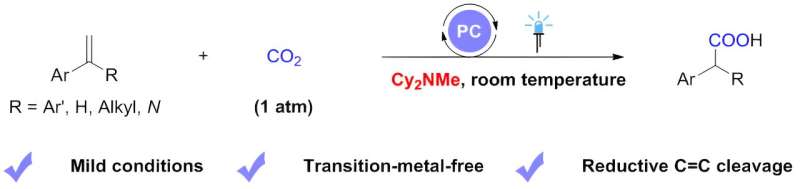
Inspired by these elegant works, with tertiary alkylamines as scission reagents for reductive C=C bond cleavage, a research team led by Prof. Da-Gang Yu from Sichuan University (China), working together with Dr. Li-Li Liao from Chongqing University (China), reported a visible-light photoredox-catalyzed carboxylation of styrenes with CO2 via reductive C=C bond cleavage. In this work, they use dicyclohexylmethylamine as an electron donor.
The success of this reaction hinges on the efficiency of dicyclohexylmethylamine to quench the excited-state photocatalyst, as well as its participation in the amino-alkyl-carboxylation reaction of olefins without significantly inhibiting the reductive quenching of the excited-state photocatalyst by the amino-alkylation intermediates.
A range of aryl olefins with various substitutions can undergo carboxylation with CO2, leading to the efficient and selective construction of a series of arylacetic acid analogs with diverse functional groups.
The method is characterized by mild reaction conditions (1-atmosphere pressure, room temperature), good tolerance of functional groups, and can be used to synthesize derivatives of drugs. In addition, this work demonstrates that the aminomethyl carboxylation intermediate, the benzyl radical, and the benzyl carbon anion are all key intermediates in the reaction. This is supported by mechanistic studies such as deuterium substitution experiments and DFT calculations.
The findings are published in the Chinese Journal of Catalysis.
More information:
Ke-Gong Cao et al, Photocatalytic carboxylation of styrenes with CO2 via C=C double bond cleavage, Chinese Journal of Catalysis (2024). DOI: 10.1016/S1872-2067(23)64583-8
Provided by
Chinese Academy of Sciences
Citation:
Photocatalytic synthesis of arylacetic acid via C=C double bond cleavage with carbon dioxide (2024, March 15)
retrieved 15 March 2024
from https://phys.org/news/2024-03-photocatalytic-synthesis-arylacetic-acid-cc.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.

