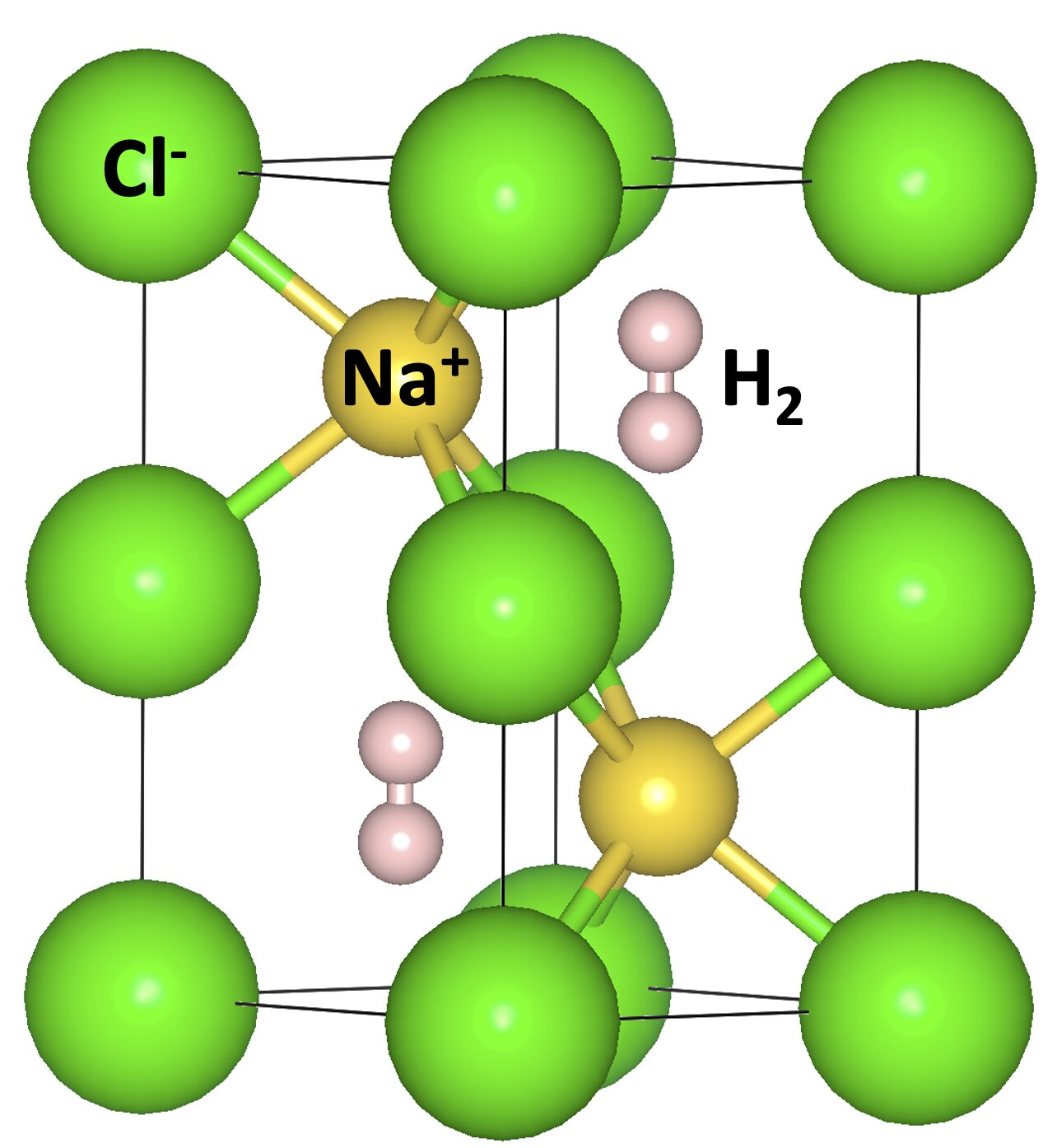
An international team has revealed a surprising universal propensity of forming stable hybrid compounds under high pressures.
The hybrid materials, which consist of inorganic components and small molecules (SM), have gained intensive attention owing to their unique chemical structure, physical properties, and potential applications. However, these unique characteristics also impose challenges on material synthesis, characterization, and the fundamental understanding of their chemical behavior. High pressure has proven to be a powerful tool for synthesizing new materials.
Under these conditions, the chemical properties of elements and the strengths of the homonuclear and heteronuclear bonds can change drastically, leading to the formation of many atypical compounds with non-intuitive compositions and structures.
The team combined crystal structure search simulations based on the swarm-intelligence algorithm as implemented in the CALYPSO program and ab initio total-energy and force calculations to systematically investigate the reactivity of many covalently bonded molecules such as H2, H2O, NH3, CH4, and CO2 with NaCl, a prototype ionic solid compound.
The calculations show that these molecules, despite whether they are homonuclear or heteronuclear, polar or non-polar, small or large, can all react with NaCl and form thermodynamically stable compounds under elevated pressures. Surprisingly, these molecules present as inserted units and maintain their chemical integrity in the new hybrid compounds.
They do not show strong chemical interactions with surrounding Na and Cl ions, despite the fact that some of the molecules are chemically very active. In contrast, the most stable molecule among all studied examples, N2, is found to transform into cyclo-N5− anions while reacting with NaCl under high pressures. It provides a new route to synthesize pentazolates, which are promising green energy materials with high energy density.
Besides providing a new route to obtain novel hybrid materials, this investigation also provides key information to the understanding of the interior structure and dynamics of many giant planets. These planets consist of both covalently bonded molecules and solid-state minerals, separated in different layers with large dispersive regions. The chemical interactions between their molecular and solid-state compositions determine their structure and dynamics.
The study is published in the journal National Science Review.
The research team included Prof. Feng Peng from Luoyang Normal University, Profs. Yanming Ma and Hanyu Liu from Jilin University, Prof. Chris Pickard from Cambridge University, and Prof. Maosheng Miao from California State. University Northridge
More information:
Feng Peng et al, Universal insertion of molecules in ionic compounds under pressure, National Science Review (2024). DOI: 10.1093/nsr/nwae016
Provided by
Science China Press
Citation:
A universal insertion of various molecules into ionic crystals under high pressure (2024, March 15)
retrieved 15 March 2024
from https://phys.org/news/2024-03-universal-insertion-molecules-ionic-crystals.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.

