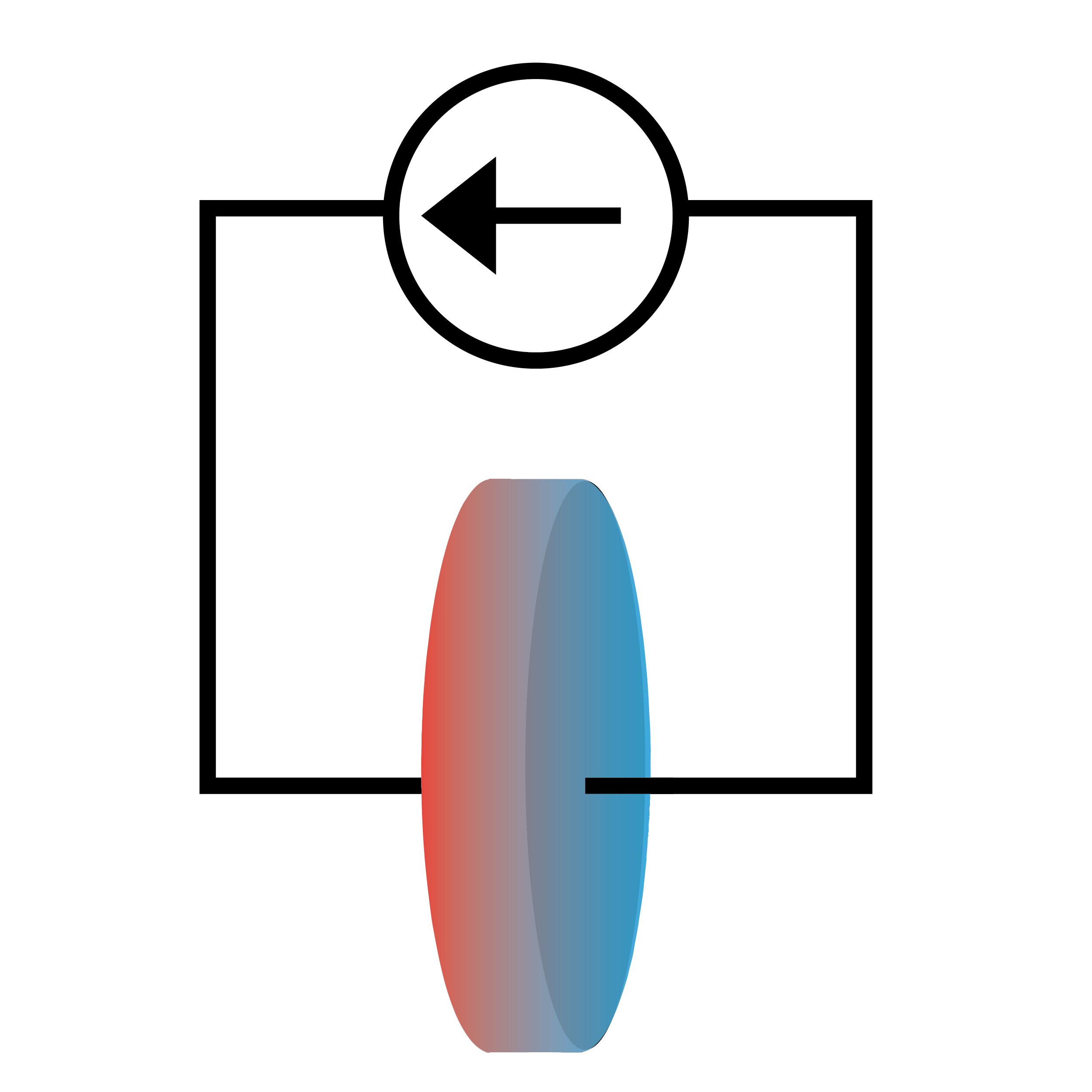
Batteries are usually studied via electrical properties like voltage and current, but new research suggests that observing how heat flows in conjunction with electricity can give important insights into battery chemistry.
A team of researchers at the University of Illinois Urbana-Champaign has demonstrated how to study the chemical properties of lithium-ion battery cells by exploiting the Peltier effect, in which electrical current causes a system to draw heat. Reported in the journal Physical Chemistry Chemical Physics, this technique allowed them to experimentally measure the entropy of the lithium-ion electrolyte, a thermodynamic feature that could directly inform lithium-ion battery design.
“Our work is about understanding the fundamental thermodynamics of dissolved lithium ions, information that we hope will guide the development of better electrolytes for batteries,” said David Cahill, a U. of I. materials science & engineering professor and the project lead. “Measuring the coupled transport of electric charge and heat in the Peltier effect allows us to deduce the entropy, a quantity that is closely related to the chemical structure of the dissolved ions and how they interact with other parts of the battery.”
The Peltier effect is well-studied in solid-state systems where it is used in cooling and refrigeration. However, it remains largely unexplored in ionic systems like lithium electrolyte. The reason is that the temperature differences created by Peltier heating and cooling are small compared to other effects.
To overcome this barrier, the researchers used a measurement system capable of resolving one hundred-thousandth of a degree Celsius. This allowed the researchers to measure the heat between the two ends of the cell and use it to calculate the entropy of the lithium-ion electrolyte in the cell.
“We’re measuring a macroscopic property, but it still reveals important information about the microscopic behavior of the ions,” said Rosy Huang, a graduate student in Cahill’s research group and the study’s co-lead author. “Measurements of the Peltier effect and the solution’s entropy are closely connected to the solvation structure. Previously, battery researchers relied on energy measurements, but entropy would provide an important complement to that information that gives a more complete picture of the system.”
The researchers explored how the Peltier heat flow changed with the concentration of lithium ions, solvent type, electrode material and temperature. In all cases, they observed that the heat flow ran opposite to the ionic current in the solution, implying that the entropy from the dissolution of lithium ions is less than the entropy of solid lithium.
The ability to measure the entropy of lithium-ion electrolyte solutions can give important insights into the ions’ mobility, governing the battery’s recharging cycle, and how the solution interacts with the electrodes, an important factor in the battery’s lifetime.
“An underappreciated aspect of battery design is that the liquid electrolyte is not chemically stable when in contact with the electrodes,” Cahill said. “It always decomposes and forms something called a solid-electrolyte interphase. To make a battery stable over long cycles, you need to understand the thermodynamics of that interphase, which is what our method helps to do.”
Zhe Cheng is the second co-lead author of the study. Beniamin Zahiri, Patrick Kwon and U. of I. materials science & engineering professor Paul Braun also contributed to this work.
More information:
Zhe Cheng et al, Ionic Peltier effect in Li-ion electrolytes, Physical Chemistry Chemical Physics (2024). DOI: 10.1039/D3CP05998G
Provided by
University of Illinois Grainger College of Engineering
Citation:
What heat can tell us about battery chemistry: Using the Peltier effect to study lithium-ion cells (2024, March 9)
retrieved 9 March 2024
from https://phys.org/news/2024-03-battery-chemistry-peltier-effect-lithium.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.