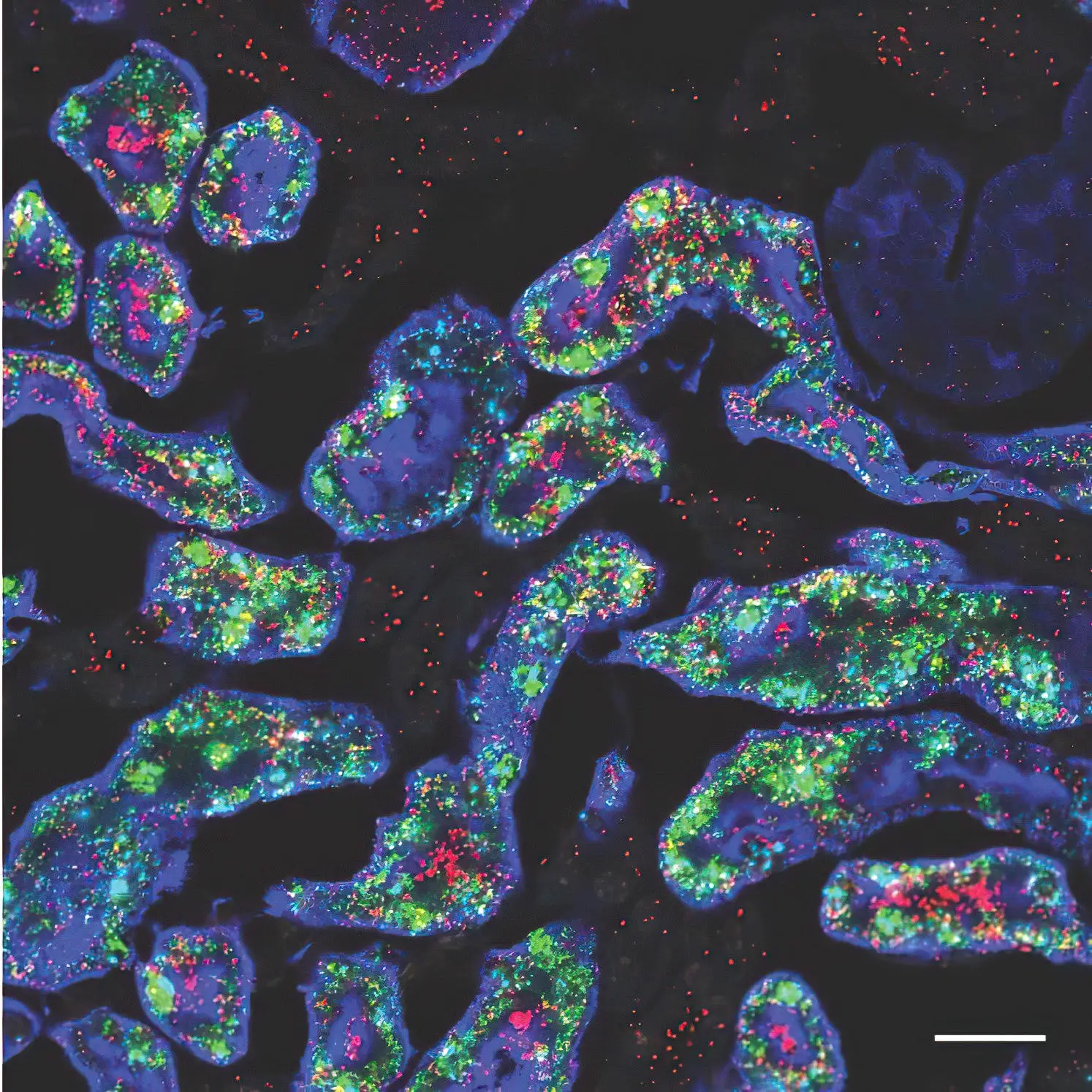

Two of the genes—Gsta4 in red and Cyp4a14 in green—that are more active in female mouse kidneys (blue). Credit: Jing Liu/McMahon Lab
Kidneys in females have been observed to be more resistant to disease and damage. However, males need not despair. Recent research led by USC Stem Cell and published in Developmental Cell sheds light on how gender hormones influence the variations in kidney resilience between male and female mice. The study also illustrates that reducing testosterone levels can “feminize” the kidney in males, potentially enhancing its capacity to withstand ailments and injuries.
“By exploring how differences emerge in male and female kidneys during development, we can better understand how to address sex-related health disparities for patients with kidney diseases,” said Professor Andy McMahon, the study’s corresponding author, and the director of the Eli and Edythe Broad Center for Regenerative Medicine and Stem Cell Research at the Keck School of Medicine of USC.
First authors Lingyun “Ivy” Xiong and Jing Liu from the McMahon Lab and their collaborators identified more than 1,000 genes with different levels of activity in male and female mouse kidneys, in a study supported by the National Institutes of Health. The differences were most evident in the section of the kidney’s filtering unit known as the proximal tubule, responsible for reabsorbing most of the nutrients such as glucose and amino acids back into the bloodstream. Most of these sex differences in gene activity emerged as the mice entered puberty and became even more pronounced as they reached sexual maturity.
Because female kidneys tend to fare better in the face of disease or injury, the researchers were interested in how the gene activity of kidneys becomes “feminized” or “masculinized”—and testosterone appeared to be the biggest culprit.
To feminize the kidneys of male mice, two strategies worked equally well: castrating males before puberty and thus lowering their natural testosterone levels, or removing the cellular sensors known as androgen receptors that respond to male sex hormones.
Intriguingly, three months of calorie restriction—which is an indirect way to lower testosterone—produced a similar effect. Accordingly, calorie restriction has already been shown to mitigate certain types of kidney injuries in mice.
To re-masculinize the kidneys of the castrated males, the researchers only needed to inject testosterone. Similarly, testosterone injection masculinized the kidneys of females who had their ovaries removed before puberty.
The scientists performed some similar experiments with mouse livers. Although this organ also displays sex-related differences, the hormones and underlying factors driving these differences are very different than those at play in the kidney. This suggests that these sex-related organ differences emerged independently during evolution.
To test whether the same genes are involved in sex-related kidney differences in humans, the scientists analyzed a limited number of male and female donor kidneys and biopsies. When it came to genes that differed in their activity between the sexes, there was a modest overlap of the human genes with the mouse genes.
“There is much more work to be done in studying sex-related differences in normal human kidneys,” said McMahon. “Given the divergent outcomes for male and female patients with kidney disease and injury, this line of inquiry is important for making progress toward eventually closing the gap on these sex-related health disparities.”
Reference: “Direct androgen receptor control of sexually dimorphic gene expression in the mammalian kidney” by Lingyun Xiong, Jing Liu, Seung Yub Han, Kari Koppitch, Jin-Jin Guo, Megan Rommelfanger, Zhen Miao, Fan Gao, Ingileif B. Hallgrimsdottir, Lior Pachter, Junhyong Kim, Adam L. MacLean and Andrew P. McMahon, 5 September 2023, Developmental Cell.
DOI: 10.1016/j.devcel.2023.08.010
Additional authors are Kari Koppitch, Jin-Jin Guo, Megan Rommelfanger, and Adam L. MacLean from USC; Zhen Miao and Junhyong Kim from the University of Pennsylvania; Fan Gao, Ingileif B. Hallgrimsdottir, and Lior Pachter from the California Institute of Technology.
One hundred percent of this work was supported by federal funding from the National Institutes of Health (grants R01DK126925 and R35GM143019) and the National Science Foundation (DMS2045327).