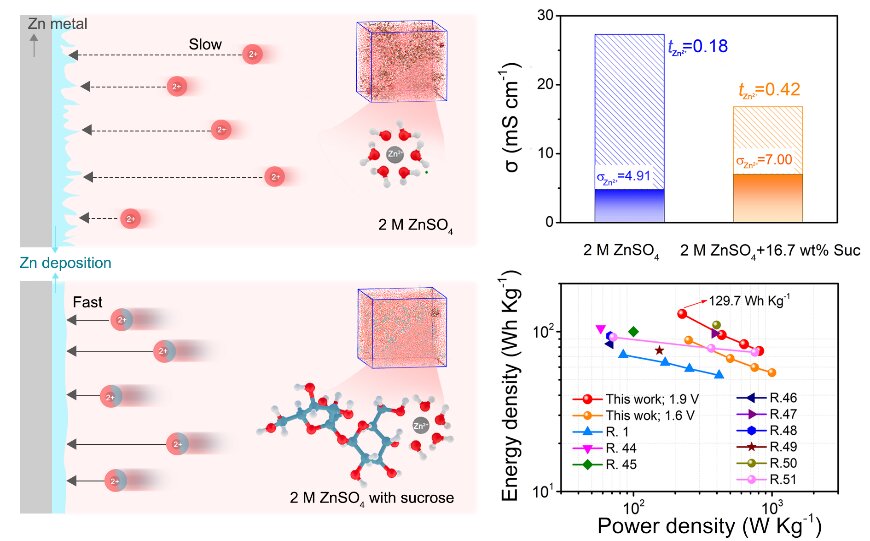
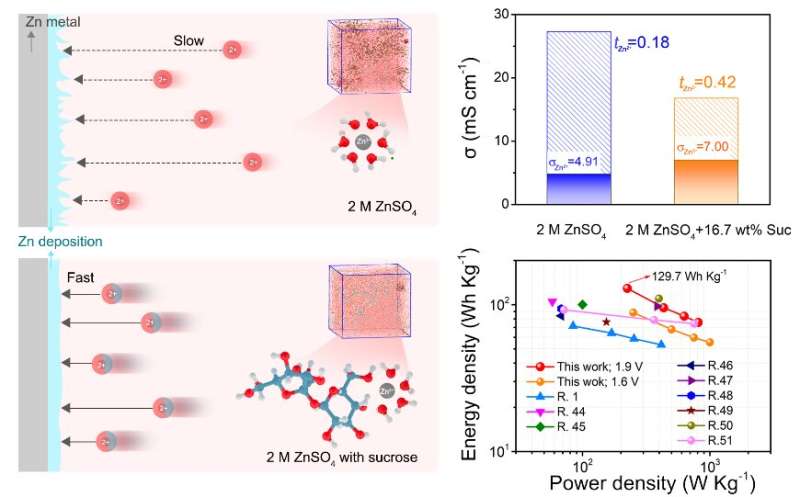
Due to their low cost and environmental friendliness, aqueous zinc batteries have the potential to play an important role in future energy storage systems for applications like power grids. However, a safety concern has slowed the progress of this emerging technology.
In a July 28 study published in Nano Research, Chinese researchers presented a solution that involves chemically modifying common table sugar to stabilize the zinc ion environment and secure future applications.
From electric cars to wind and solar power systems, an increasingly diverse range of power-hungry applications continue to boost demands for large-scale, low-cost energy storage. Aqueous Zinc (Zn) batteries quickly rose to the top as one of the more promising options for sustainably meeting the demand, according to the study.
“They are high safety and cost-effective compared to current lithium-ion batteries with flammable organic electrolytes,” said paper author Meinan Liu, associate professor of nano-tech and nano-bionics at the University of Science and Technology of China. “In addition, Zn anode presents super high theoretical capacity, which makes these Zn batteries even more promising for applications like future grid energy storage.”
However, when the zinc ion (Zn2+) concentration on the surface of the anode drops to zero, dendrites start growing. Uncontrolled Zn dendrite growth deteriorates electrochemical performance and pose a serious threat to safe operation.
“These dendrites can penetrate the separator and cause the battery to short-circuit,” Liu said.
Past studies have shown that adjusting the solvent environment (called “solvation structure”) can increase the mobility of Zn2+ in response to the electric field successfully suppresses the growth of dendrites. The problem was that these previous adjustments—like introducing other salts or including fewer water molecules—ended up decreasing the ionic conductivity of the system as well.
There was a fundamental understanding gap between Zn2+ solvation structure and its mobility, explained by Liu. This was a key factor affecting the dendrite growth and stability of Zn anode.
In attempt to bridge this gap, a collaborative research team from multiple Chinese institutions tried a new tack: introducing common table sugar with multiple hydroxyl groups (a hydrogen and an oxygen bound together) into the electrolyte to adjust solvation structure of Zn2+.
By conducting atomistic simulations and experiments, the research team confirmed that the sucrose molecules enhanced mobility and stopped dendrite growth without compromising stability. In fact, this method provided unlooked-for benefits as well:
“Findings confirm that sucrose molecules in the solvation sheath not only enhance the mobility, ensuring fast Zn2+ kinetics, but also protects the Zn anode from water corrosion and successfully achieves Zn dendrite-free deposition and side reaction suppression,” Liu said.
This demonstrates the great potential of using this simple sucrose-modification for future high-performance zinc batteries and brings the research field a step closer to the ultimate goal of achieving a safe, green, high-performance Zn battery.
“Hopefully this safe, low-cost Zn battery could be applied in grid energy storage,” Liu said.
This technique also lends itself to additional variations and modifications: Zn-carbon cells deliver higher energy density and improved stability, suggesting a great potential application of sucrose-modified electrolytes for future Zn batteries.
In future studies, the researchers will also be considering possible use cases and roadblocks for aqueous zinc batteries, specifically how they might handle extreme temperatures.
“The aqueous electrolyte of Zn battery will be frozen in low temperature, so we are looking into how to address the temperature influence on battery performance,” Liu said.
Scientists develop low-temperature resisting aqueous zinc-based batteries
Yufang Cao et al, Fast Zn2+ mobility enabled by sucrose modified Zn2+ solvation structure for dendrite-free aqueous zinc battery, Nano Research (2022). DOI: 10.1007/s12274-022-4726-3
Provided by
Tsinghua University Press
Citation:
Common table sugar key to allaying safety concern in aqueous zinc batteries (2022, July 29)
retrieved 29 July 2022
from https://phys.org/news/2022-07-common-table-sugar-key-allaying.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.