The lab of Kathy Gould, Louise B. McGavock Professor and professor of cell and developmental biology, used a multi-disciplinary approach that included structural biology, biochemistry, and molecular biology to investigate the regulation of the CK1 enzyme family. The research, led by Sierra Cullati, a postdoc in the Gould lab, and carried out in conjunction with Jun-Song Chen, research assistant professor of cell and developmental biology, and scientists from Goethe University and the Structural Genomics Consortium in Frankfurt, Germany, and from Harvard University, was published in Molecular Cell.
CK1 enzymes are a family of multifunctional kinases—enzymes that can phosphorylate, or add phosphate groups to, other proteins—that are critical for several cellular functions including DNA repair, endocytosis, and mitotic checkpoint signaling. Regulation of CK1 enzymes is exceptionally important as dysfunction of these enzymes contributes to several conditions that include cancer, neurodegenerative diseases, and sleep disorders.
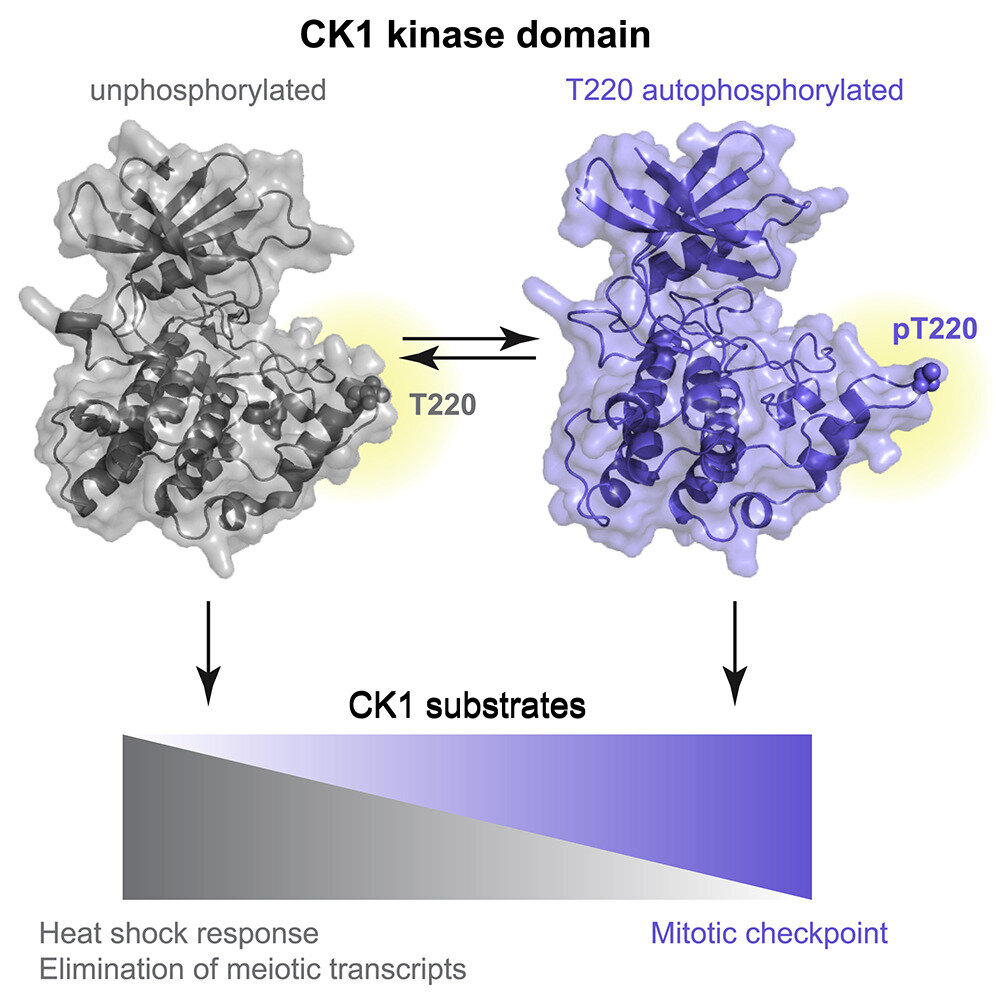
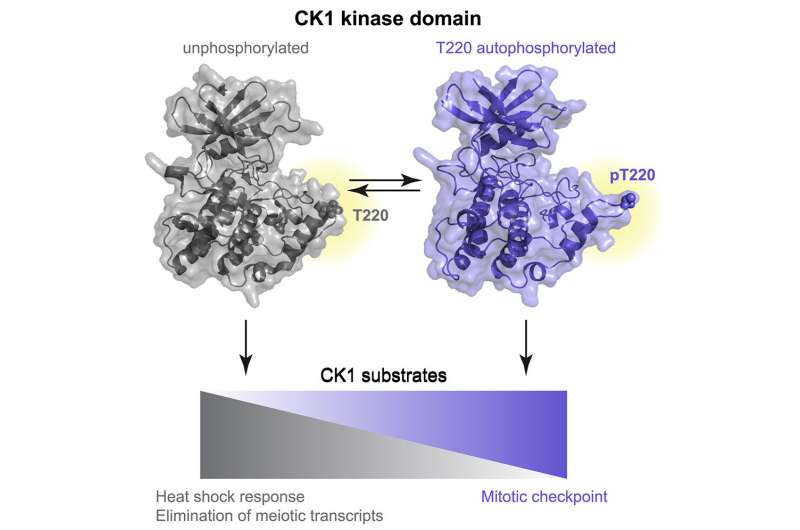
There are seven CK1 enzymes in mammals that perform different functions, but they are highly conserved in their catalytic domain, the region responsible for phosphorylation. Gould and colleagues found that one mechanism of CK1 activity, and thus one mechanism of regulation, is the self-phosphorylation of a conserved amino acid residue in its catalytic domain.
The researchers further investigated how this self-phosphorylation regulates activity and discovered that phosphorylation at this site altered the substrate specificity of CK1 enzymes. Substrate specificity refers to the determination of which other proteins the CK1 kinases will phosphorylate, which in turn determines which pathways within a cell get activated. In general, the phosphorylation state of CK1 enzymes controls their function—or dysfunction—within a cell. Determining which pathways are controlled by the phosphorylated versus non-phosphorylated states of the enzymes is a step toward the development of better treatments with fewer side effects for the diseases caused by enzyme dysfunction.
The Gould lab and collaborators hope to build upon this work by determining other sites of CK1 self-phosphorylation and investigating the pathways they regulate; there are several potential self-phosphorylation sites clustered together on one end of the protein, for example, that intrigue the researchers. Additionally, they plan to investigate how the discovered phosphorylation sites work together to provide additional control under different cellular conditions, such as cellular stress.
New discovery on regulation of organelle contacts
Sierra N. Cullati et al, Kinase domain autophosphorylation rewires the activity and substrate specificity of CK1 enzymes, Molecular Cell (2022). DOI: 10.1016/j.molcel.2022.03.005
Vanderbilt University
Citation:
Self-regulation of an enzyme with critical cellular functions (2022, May 9)
retrieved 9 May 2022
from https://phys.org/news/2022-05-self-regulation-enzyme-critical-cellular-functions.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.