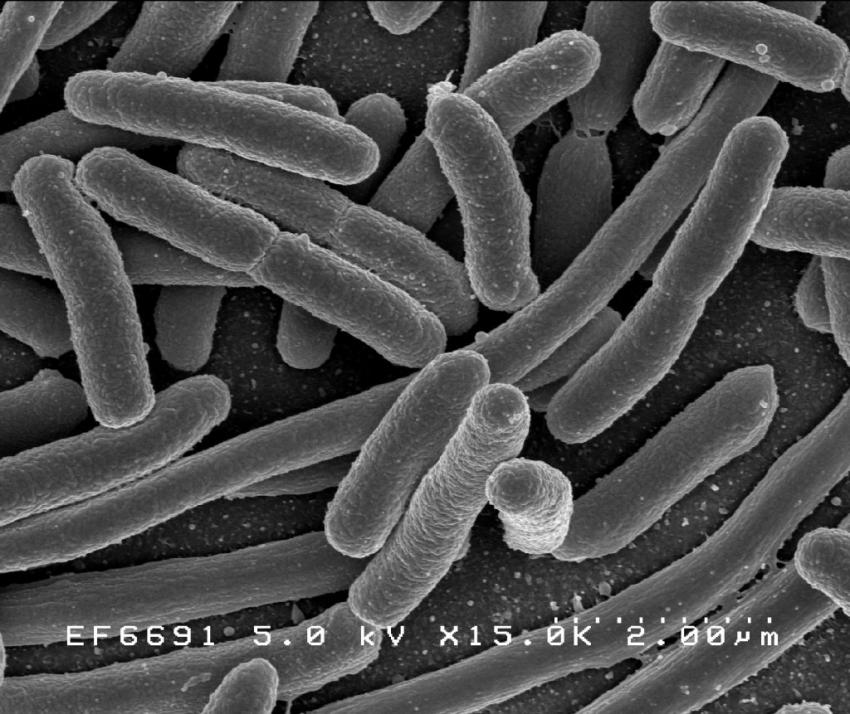
Humans are colonized with thousands of bacterial strains. Researchers are now focused on genetically modifying such bacteria to enhance their intrinsic therapeutic properties.
One goal is to develop smart microbes that release therapeutic payloads at sites of disease, thus maintaining therapeutic efficacy while limiting many of the side effects that can be associated with the systemic administration of conventional drugs.
Investigators at Massachusetts General Hospital (MGH), a founding member of Mass General Brigham (MGB), have engineered a strain of the probiotic Escherichia coli (E. coli), Nissle 1917, to secrete proteins of therapeutic value into its surroundings.
When engineered to secrete an antibody that blocks inflammation, this “smart microbe”, was as efficacious as a systemically delivered antibody, the mainstay of current therapy, in limiting the development of colitis in a mouse model of inflammatory bowel disease (IBD).
The work is described in the newest issue of Cell Host & Microbe.
One of the challenges of enhancing the therapeutic capabilities of this beneficial microbe was to enable it to secrete proteins into its surroundings. E. coli are surrounded by an outer envelope across which few proteins are transported.
“Many pathogenic relatives of E. coli directly transport bacterial proteins across their outer envelope into human cells using a syringe-like machine,” says senior author Cammie F. Lesser, MD, Ph.D., a physician-scientist in the Infectious Disease Division at MGH, associate professor of Medicine at Harvard Medical School and d’Arbeloff MGH Research Scholar.
Lesser’s lab at MGH has been studying these complex protein secretion systems for more than two decades with the ultimate goal of reengineering them as drug delivery systems.
Using knowledge gained from fundamental-based research, the lab introduced a version of this secretion machine into beneficial E. coli and modified it to secrete proteins into its surroundings.
They also engineered a variety of proteins of therapeutic value to be recognized as secreted proteins of this machine. The resulting programmable platform is referred to as PROT3EcT for probiotic type III secretion E. coli.
As proof of the potential therapeutic value of PROT3EcT, Lesser and her colleagues tested the engineered E. coli in a mouse model of inflammatory bowel disease.
PROT3EcT that was engineered to secrete nanobodies that bind to and inhibit tumor necrosis factor (TNF) alpha, a pro-inflammatory cytokine, was as effective in blocking the development of inflammation in the intestines of mice as an injected monoclonal antibody that targets the same cytokine.
Monoclonal antibodies that neutralize TNF alpha result in general suppression of the immune system, which can have unintended effects.
“Patients administered these drugs systemically are at risk for developing life-threatening infections as well as lymphoma,” says Lesser. “By using engineered bacteria, it should be possible to deliver these anti-inflammatory antibodies and limit immunosuppression directly to where inflammation is present.”
Lesser and her colleagues are now working on engineering bacterial strains that will secrete therapeutic proteins in response to specific conditions, such as when inflammation begins developing in the gut.
Engineered E. coli can also be outfitted to secrete antibodies that block toxins released by harmful strains of bacteria. Lesser’s team is investigating the microbe’s potential to treat intestinal infections such as Clostridiodes difficile (C. diff), colitis, and other toxin-driven infections.
E. coli and other bacteria also replicate in solid tumors, so Lesser and others are researching the use of engineered microbes as anti-cancer agents. “We hope to advance these strains towards the treatment of a variety of human diseases by outfitting them to secrete a variety of proteins of therapeutic value,” says Lesser.
Co-authors include Jason P. Lynch, Coral Gonzalez-Prieto, Analise Z. Reeves, John M. Leong, Charles B. Shoemaker, and Wendy S. Garrett.
More information:
Jason P. Lynch et al, Engineered Escherichia coli for the in situ secretion of therapeutic nanobodies in the gut, Cell Host & Microbe (2023). DOI: 10.1016/j.chom.2023.03.007
Provided by
Massachusetts General Hospital
Citation:
Engineered E. coli delivers therapeutic nanobodies to the gut (2023, March 31)
retrieved 31 March 2023
from https://phys.org/news/2023-03-coli-therapeutic-nanobodies-gut.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.