A groundbreaking technique developed by researchers affiliated with the USC Michelson Center for Convergent Bioscience presents a new way of gathering and organizing highly detailed information about organic tissues in record time.
The methods could someday be used to rapidly process tissue biopsies in cancer care or detecting bacteria in food processing plants.
Tissues emit signals, or intrinsic fields, that while detectable are very weak and hard to differentiate. The technique, detailed in a pair of papers published in Nature Methods and Cell Reports Methods, uses a complex mathematical algorithm to improve the quality of the signals and then separate them.
The new technique is comparable to how a streaming service presents different levels of compression to ensure their video is consistent regardless of a user’s internet connection, according to Francesco Cutrale, co-principal investigator and research assistant professor at the USC Viterbi School of Engineering.
“Based on how fast your connection is, the streamer will send the video with different levels of compression that is then recomposed optimally for your device,” he said. “We’re doing something similar: We’re taking very large, very complex data and moving it into a space where it is compressed. We can then look at very large data sets—associated by similarity into an enormous histogram—and analyze this data in record time and with very high sensitivity.”
A window into the complexity of cells and organic tissue
The algorithm—detailed in Nature Methods earlier this year—continues the recent refinement of high-content imaging approaches utilizing fluorescence. Thanks to its high contrast and specificity, as well as its adaptability, fluorescence has enabled the detection and defining of specific molecules. However, these newer techniques don’t work for imaging live, or in vivo, samples because these approaches have limited sensitivity and may damage specimens.
In the paper, the research team showed how the technique, called Hybrid Unmixing, could be used to analyze live organic tissue cleanly and efficiently. The technique uses linear unmixing, a method to analyze different components within a specimen marked by chemical compounds called fluorophores.
They then visualize these components using hyperspectral phasors, which use the entire color spectrum, rather than solely red, blue and green. In doing so, Hybrid Unmixing allows for simultaneous imaging of bright and dim labeled components within organic tissue, even under low illumination.
Allowing concurrent analysis of the cellular behaviors and cellular metabolism of these labeled components, the technique will provide more accurate insights into the complexity of biological systems.
“There’s a push in the research space for understanding complex biological systems,” Cutrale said.
“While researchers typically examine only two or three labels at once, the truth is that there are more than just a few factors interacting within cells. The challenge is that these signals often appear very similar, making them difficult to distinguish. In our paper, we have successfully identified and separated up to 14 different signals. This breakthrough will provide researchers with a more comprehensive understanding of the activity inside cellular and biological systems.”
The algorithm provides the foundation for numerous applications from an industry point of view, Cutrale said.
“We work in the life sciences, but it’s easy to imagine numerous applications to evaluate the quality of fruits, the presence of pesticides or how to optimize production in many other fields,” he said.
SHy-Cam offers low-cost, high-quality imaging tool
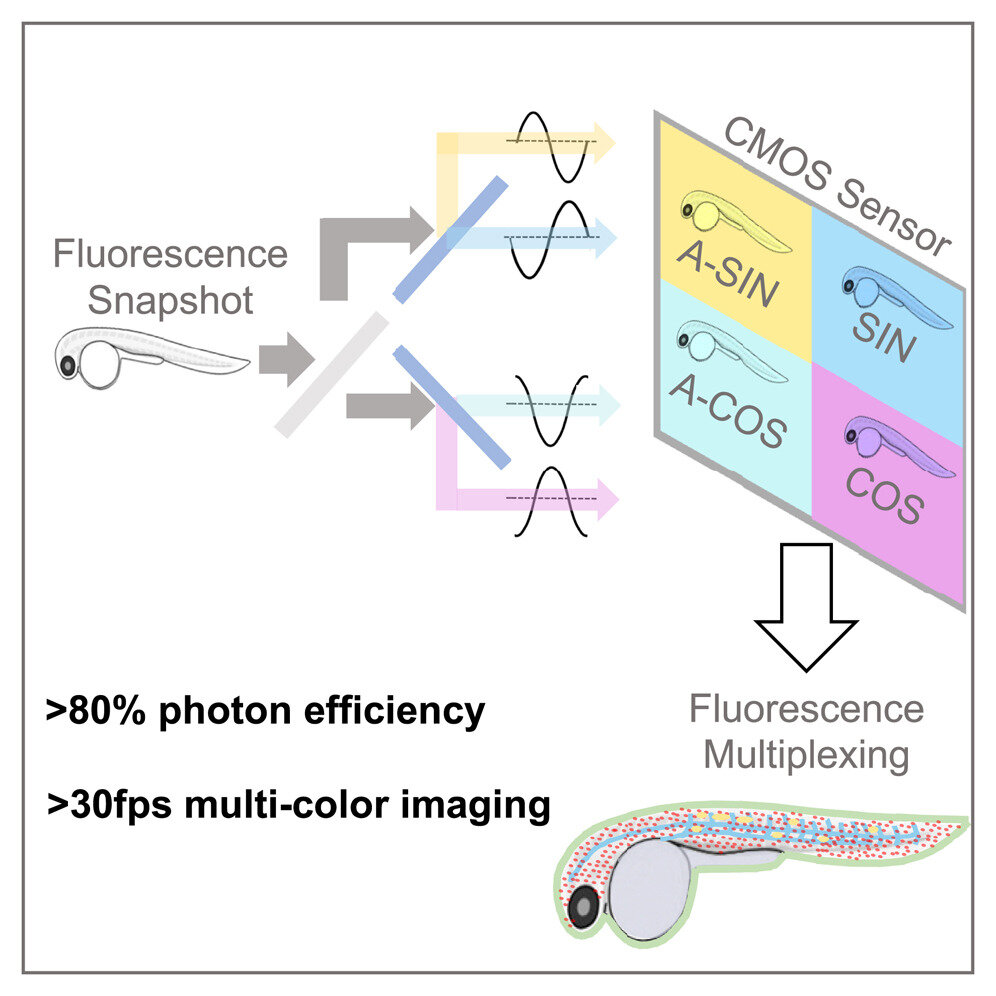
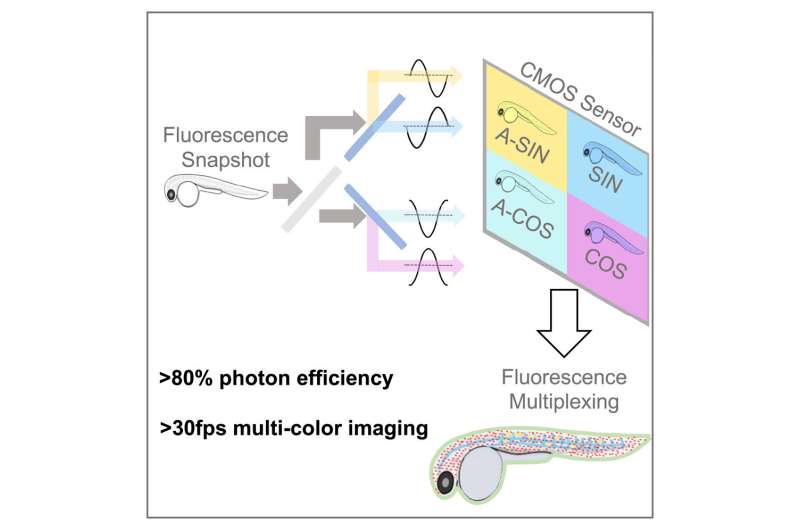
A subsequent paper, published today in Cell Reports Methods, describes hardware—dubbed SHy-Cam, short for Single-shot Hyperspectral Phasor Camera—designed by the research team optimized to capture this type of information. Typical tissue-imaging techniques utilizing fluorescence use color channels across the spectrum to compensate for overlap between labels. This technique reduces imaging speed and, if exposed to excessive light, can ultimately damage the samples.
With the SHy-Cam, the researchers were able to use the new algorithm to obtain spectral information quickly and efficiently, in a camera that can be built with already available optical components. The new equipment described in the paper is capable of acquiring 30 data sets per second, with a photon efficiency of over 80%. This makes it a powerful tool for multi-color, in-vivo imaging, the researchers said.
“How do you produce a two-dimensional picture with a 2D sensor? You take a picture,” Cutrale said. “Our challenge is how to capture a 3D data set with a 2D sensor. A typical color sensor acquires three colors—red, blue and green—or it receives everything through its grayscale sensors.
“In our case, we need to request 42 channels of information—that’s not common, nor is it efficient. We designed in this paper a new approach that can obtain an encoded version of the spectral information with a single image.”
Cutrale said they do this by utilizing light. The team harnessed light to transform the information and used it to perform the calculations before compressing it on to the sensor. In using this approach, the team showed how it can receive the entire spectrum and the dimensions of the image.
“We’ve captured the X- and Y-axes of the image—its height and width—and also the spectral information on the wavelength-axis, all together in a single image with a standard camera,” he said. “That’s quite a powerful approach. We obtained efficiencies in this hardware approach which are in some cases up to eight times faster than existing instrumentation. In other words, eight times more light reaches the camera sensor in this compressed fashion.”
More information:
Francesco Cutrale, a Single-shot Hyperspectral Phasor Camera for fast, multi-color fluorescence microscopy, Cell Reports Methods (2023). DOI: 10.1016/j.crmeth.2023.100441. www.cell.com/cell-reports-meth … 2667-2375(23)00056-5
Hsiao Ju Chiang et al, HyU: Hybrid Unmixing for longitudinal in vivo imaging of low signal-to-noise fluorescence, Nature Methods (2023). DOI: 10.1038/s41592-022-01751-5
Provided by
University of Southern California
Citation:
Pictures inside a cell: Researchers develop new tool to provide greater insight into biological processes (2023, March 31)
retrieved 31 March 2023
from https://phys.org/news/2023-03-pictures-cell-tool-greater-insight.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.