
This story is part of CBC Health’s Second Opinion, a weekly analysis of health and medical science news emailed to subscribers on Saturday mornings. If you haven’t subscribed yet, you can do that by clicking here.
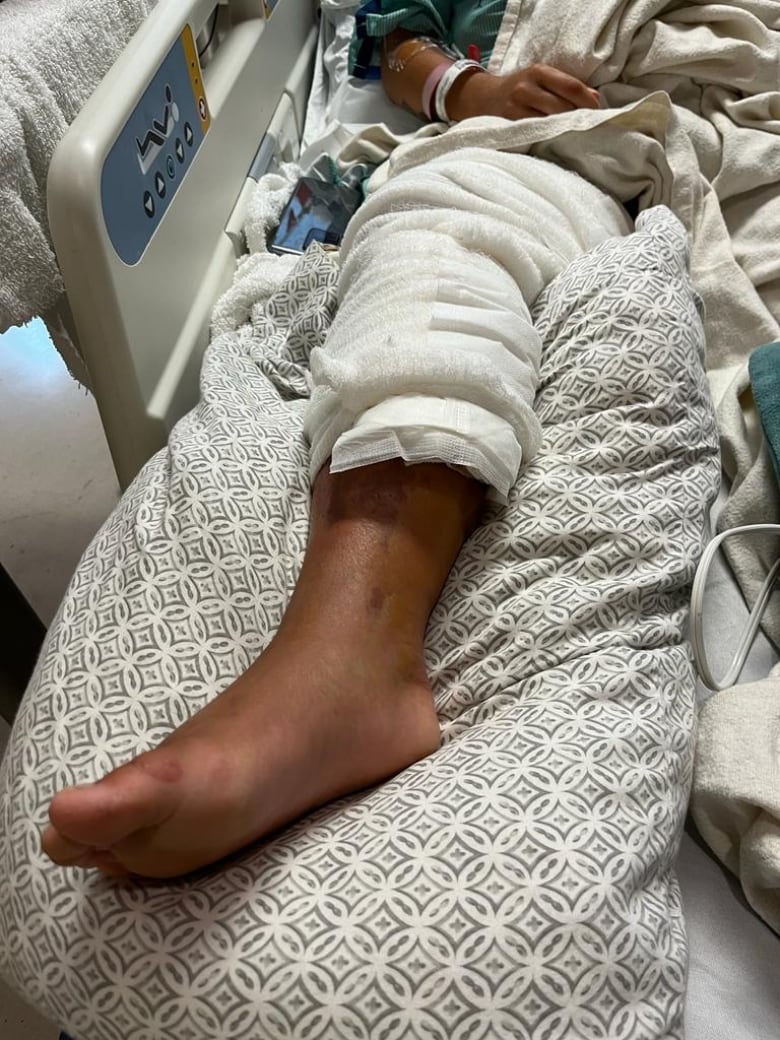
Melissa Murray regularly ran 10 kilometres a day — until a serious bacterial infection caused her to nearly lose a leg.
Last summer, the Toronto woman who’d worked 60-hour weeks as an account manager suddenly needed round-the-clock care to recover from sepsis. The life-threatening condition results from the immune system’s overreaction to fighting an infection.
Septic shock deprived Murray’s heart and kidneys of vital blood and oxygen, and her blood pressure plunged dangerously low. To save her life, surgeons urgently had to cut out half a calf muscle and tendons plus the inner side of the other leg.
“I kept saying it feels like there’s like a campfire in my leg,” Murray, 46, recalled of the “excruciating” ordeal in July 2023.
“The pain was so bad I wanted to get out of my body.”
Murray had an invasive Group A strep (iGAS) bacterial infection that could not be treated with standard antibiotics. Doctors don’t know how Murray got the infection, saying it could’ve been from something as small as a nick in the skin from shaving.
Antibiotic-resistant microbes, sometimes called superbugs, are major contributors to sepsis — and may well be the biggest international public health threat of our time. World leaders called antibiotic resistance “an urgent global health threat.” Here’s what patients and doctors say should change.
An urgent global threat
Bacteria live in or on us, often beneficially or harmlessly. But antimicrobial resistance occurs when the germs that can cause infections develop the ability to evade medicines like antibiotics.
At this week’s United Nations General Assembly in New York, world leaders called antimicrobial resistance or AMR “an urgent global health threat,” and aimed to reduce the estimated 4.95 million human deaths associated with it per year by 10 per cent by 2030.
As bacteria evolve over time, they can stop responding to the antibiotics that once killed them. Infections are then harder to treat — and sometimes even impossible.
Sepsis is one of the devastating consequences of antimicrobial resistance. The resistance can also render a minor skin wound incurable or turn a routine surgery into an opportunity for dangerous microbes to invade.
Antimicrobial resistance “is one of the major causes of death in [all] our countries as we speak, but the worst part of the news is that it will be the No. 1 cause of death by 2050,” Barbadian Prime Minister Mia Mottley, the chair of the Global Leaders Group on AMR, told reporters from the UN on Thursday.
Antibiotics are a precious resource. Bacteria acquire resistance genes from each other. If someone doesn’t take all of the antibiotic pills they’ve been prescribed, a single bacterium remaining in the person’s system can evolve resistance and quickly pass on the advantage.
Dr. Alison Fox-Robichaud, scientific director of Sepsis Canada, witnesses the links between antimicrobial resistance and sepsis in the intensive care unit where she works.
“We can often cure the infection,” said Fox-Robichaud, a professor of medicine at McMaster University. “It takes longer to cure the sepsis, the results from that infection. But it means we may have less antibiotics to use for those serious bacteria that have acquired all of those resistance genes.”
Fox-Robichaud said in Canada, 80 per cent of people with serious infections are identified in the emergency department, meaning they picked up microbes in the community where they live. And many infections acquired in intensive care units are resistant to antibiotics, because of overuse of the drugs, the long stays of patients who are highly susceptible to such infections due to co-occurring illnesses, as well as invasive procedures like placing urinary catheters.
“It’s frustrating because I know we’ve got vaccine-preventable infections,” Fox-Robichaud said, listing flu and pneumonia as well as RSV in older adults as examples. “If people took them, I wouldn’t be seeing as many people in my ICU.”
‘Like 3 tennis balls underneath my skin’
For Murray, the iGAS infection initially weakened her from vomiting, followed a day later by diarrhea that kept her up overnight. Her temperature hit 40 C and persisted all day, despite her taking fever-reducing pills.
When the sun rose, Murray said her legs felt weirdly hot: “It just looked like three tennis balls underneath my skin,” she said.
Murray called her boyfriend into the room and they headed to the emergency department.
She learned the hot, balled skin was from cellulitis, a common but potentially serious bacterial skin infection. She said the pain of the infection was “thousands” times worse than what she’d experienced in childbirth.
After 13 days in hospital, Murray went home with a cocktail of IV antibiotics that doctors nicknamed the “Melissa blend.”
Antibiotics prescribed too blindly, surgeon says
Ara Darzi of Imperial College London is a surgeon who attended the UN meeting. He said he’d like the world to adopt a new goal by 2030: Diagnose the type of bacteria that’s causing the infection before prescribing any antibiotic.
“The supply of new antibiotics is not keeping up with demand,” Darzi said in an email to CBC News. But for this approach to work, new therapeutics and vaccines, as well as new diagnostic kits need to be developed and made widely available.
“We wouldn’t dream of giving chemotherapy without knowing the type of cancer — why do we tolerate this with infections?”
Thea Turcotte says her life was likely saved by an experimental treatment developed in Winnipeg for her periprosthetic joint infection.
Mathieu Poirier, an assistant professor of social epidemiology at Toronto’s York University, notes antimicrobial resistance in bacteria directly caused over a million deaths in 2021 globally, ranging from common infections like pneumonia to untreatable sepsis or tuberculosis.
Preventing infections through access to safe water and sanitation as well as improving vaccination rates are important steps to curbing antimicrobial resistance, he said.
Poirier said that in North America and Europe, people have been using antibiotics and other antimicrobials at far higher rates than elsewhere in the world: “In many ways we are contributing more to the problem, while not even dealing with the worst of the consequences.”
For Murray, the ongoing consequences of her infection, which occurred a year ago, include splotches of sore skin on her shoulders, and a leg she must often keep elevated because of swelling. But she can walk now, with a cane, and can now drive short distances.
“I like to think I’m thriving because I’m alive.”
