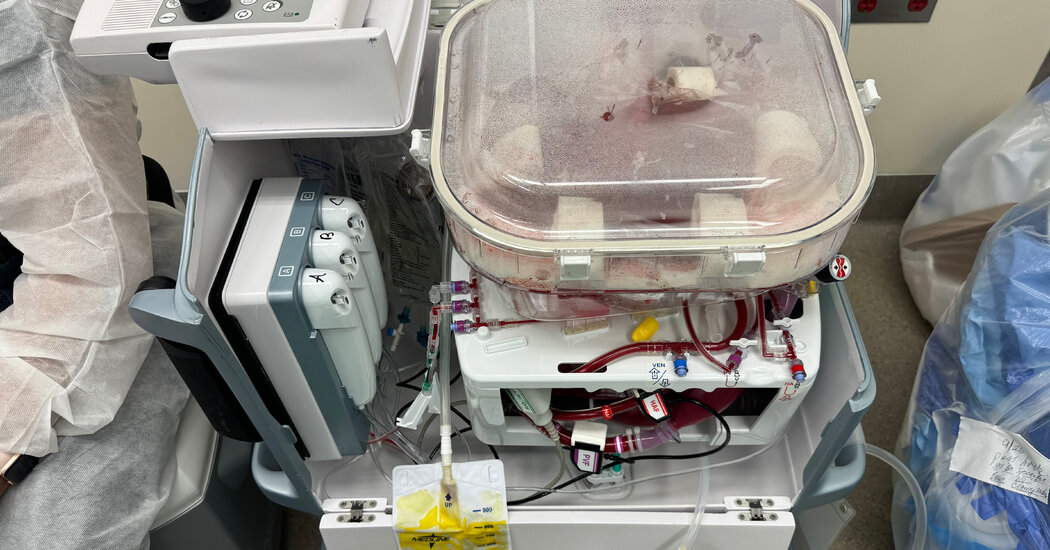
On some level, the human liver in the operating room at Northwestern Memorial Hospital in Chicago was alive. Blood circulating through its tissues delivered oxygen and removed waste products, and the organ produced bile and proteins that are essential to the body.
But the donor had died a day earlier, and the liver lay inside a boxy plastic device. The organ owed its vitality to this machine, which was preserving it for transplantation into a needy patient.
“It’s a little bit science fiction,” said Dr. Daniel Borja-Cacho, a transplant surgeon at the hospital.
Surgeons are experimenting with organs from genetically modified animals, hinting at a future when they could be a source for transplants. But the field is already undergoing a paradigm shift, driven by technologies in widespread use that allow clinicians to temporarily store organs outside the body.
Perfusion, as its called, is changing every aspect of the organ transplant process, from the way surgeons operate, to the types of patients who can donate organs, to the outcomes for recipients.
Most significantly, surgical programs that have adopted perfusion are transplanting more organs.
Since 2020, Northwestern has had a 30 percent uptick in its volume of liver transplants. Nationally, the number of lung, liver and heart transplants each rose by more than 10 percent in 2023, one of the largest year-over-year increases in decades.
Without blood flow, organs rapidly deteriorate. That’s why clinicians have long considered the ideal organ donor to be someone who died under circumstances that ended brain activity but whose heart continued beating, keeping the organs viable until they could be matched with recipients.
To minimize injury to organs after their removal from a donor’s blood supply and before they are connected to a recipient’s, surgeons used to cool them to just above freezing, significantly slowing their metabolic processes.
This extends the window in which organs can be transplanted, but only briefly. Livers remain viable for no longer than 12 hours, and lungs and hearts closer to six.
Scientists have long experimented with techniques for keeping organs in more dynamic conditions, at a warmer temperature and perfused with blood or another oxygenated solution. After years of development, the first device for preserving lungs via perfusion won approval from the Food and Drug Administration in 2019. Devices for perfusing hearts and livers were approved in late 2021.
The devices essentially pump blood or an oxygenated fluid through tubes into the blood vessels of the donated organ. Because cells in a perfused organ continue to function, clinicians can better assess whether the organ will thrive in a recipient’s body.
Bolstered by that information, transplant surgeons have begun to use organs from older or sicker donors that they might otherwise have turned down, said Dr. Kris Croome, a professor of surgery at the Mayo Clinic in Florida. “We’re going after organs we never would have before, and we’re seeing good outcomes,” he said.
Perfusion also eases the grueling process of organ recovery and transplant, hourslong surgeries that doctors often conduct against the clock, beginning in the middle of the night and completed in back-to-back succession.
Now surgical teams can recover an organ, perfuse it overnight while they sleep and complete the transplant in the morning without fear that the delay will have damaged the organ.
Perhaps most important, perfusion has further opened the door to organ donation by comatose patients whose families have withdrawn life support, allowing their hearts to eventually stop. Each year, tens of thousands of people die this way, after the cessation of circulation, but they were rarely donor candidates because the dying process deprived their organs of oxygen.
Now, surgeons are perfusing these organs, either by removing them to a machine or, in a lower-tech manner, by recirculating blood in that region of the donor’s body. And that has made them much more appealing for transplant.
Since 2020, the number of livers transplanted after the circulatory death of the donor has doubled, according to an analysis of data from the United Network for Organ Sharing, the nonprofit that runs the United States’ transplant system.
Once, surgeons never used hearts from such donors because of that organ’s sensitivity to oxygen deprivation; in 2023, thanks to perfusion, they transplanted over 600.
By tapping this new cadre of donors, transplant centers said they could find organs more quickly for the excess of patients in urgent need. Dr. Shimul Shah said the organ transplant program he directs at the University of Cincinnati had essentially wiped out its waiting list for livers. “I never thought, in my career, I would ever say that,” he said.
One obstacle to the adoption of the technology may be cost. At the rates currently demanded by device makers, perfusing an organ outside the body can add more than $65,000 to the price of a transplant; smaller hospitals may not be able to justify the upfront expense.
One of the leading companies, TransMedics, raised its prices substantially after regulators approved its device, prompting a stern letter from Representative Paul Gosar, Republican of Arizona, who wrote: “What began as a promising medical equipment innovation and an opportunity to increase transplantation nationwide is now being held hostage by a public company that has lost its true north.”
But some surgeons said that the technology might nonetheless save money, since patients who receive perfused organs generally leave the hospital quicker and with fewer complications, and have better medium- and long-term outcomes.
Surgeons are still exploring the upper limits of how long perfused organs can survive outside the body, and as substantially as the technologies are already altering transplant, some say this is only the beginning.
Dr. Shaf Keshavjee, a surgeon at the University of Toronto whose lab was at the forefront of developing technologies to preserve lungs outside the body, said the devices could eventually allow doctors to remove, repair and return lungs to sick patients rather than replace them. “I think we can make organs that will outlive the recipient you put them in,” he said.
Dr. Ashish Shah, the chairman of cardiac surgery at Vanderbilt University, one of the busiest heart transplant programs in the country, agreed, calling that “the holy grail.”
“Your heart sucks,” he said. “I take it out. I put it on my apparatus. While you don’t have a heart, I can support you with an artificial heart for a little while. I then take your heart and fix it — cells, mitochondria, gene therapy, whatever — and then I sew it back in. Your own heart. That’s what we’re really working for.”