It was officially described 115 years ago. But billions of pounds and hundreds of failed trials later, scientists still haven’t been able to crack one of the world’s most devastating diseases — Alzheimer’s.
Curing the cruel memory-robbing disorder is considered one of the Holy Grails of medicine but it has alluded scientists for decades. Currently, the only approved drugs for Alzheimer’s merely dampen some of the symptoms — and only temporarily — but do not stop the disease from progressing.
But there is some tentative optimism among experts who feel they are finally edging closer. Dr Mark Dallas, a neuroscientist from the University of Reading, told MailOnline he hopes a big breakthrough could come within the next decade.
On Tuesday, scientists found giving dementia patients ADHD drugs like Ritalin, normally prescribed to hyperactive children, can improve memory, learning and reduce apathy – one of the sad symptoms of Alzheimer’s that often sees patients cut themselves off emotionally from loved ones.
The British researchers who found the link after reviewing existing research believe the drugs help kickstart the brain region responsible for cognition — and they are calling for new clinical trials.
Elsewhere, new therapies are being trialled that involve injecting people with stem cells that can mimic brain tissue to regrow parts of the organ damaged by the disease.
There is also optimism about antibody jabs that train the body to recognise the early signs of Alzheimer’s. They are already being trialled in humans and several antibody cocktails were rapidly developed and approved for Covid during the pandemic.
Other teams are studying zapping the brain with light or using oxygen therapy in an attempt to reverse the damage caused by the disease.
These potential treatments are still years away from being rolled out on a mass scale, even if they are found to work. But in the meantime, scientists are looking at whether any existing medications can be repurposed to fight the memory robbing disorder.
MailOnline looks at some of the potential breakthroughs that could one day lead to curing or reversing of Alzheimer’s:




Vaccines and antibodies, brain zapping helmets, oxygen therapy and stem cells are just some of the areas experts are exploring in the hunt for a cure for Alzheimer’s
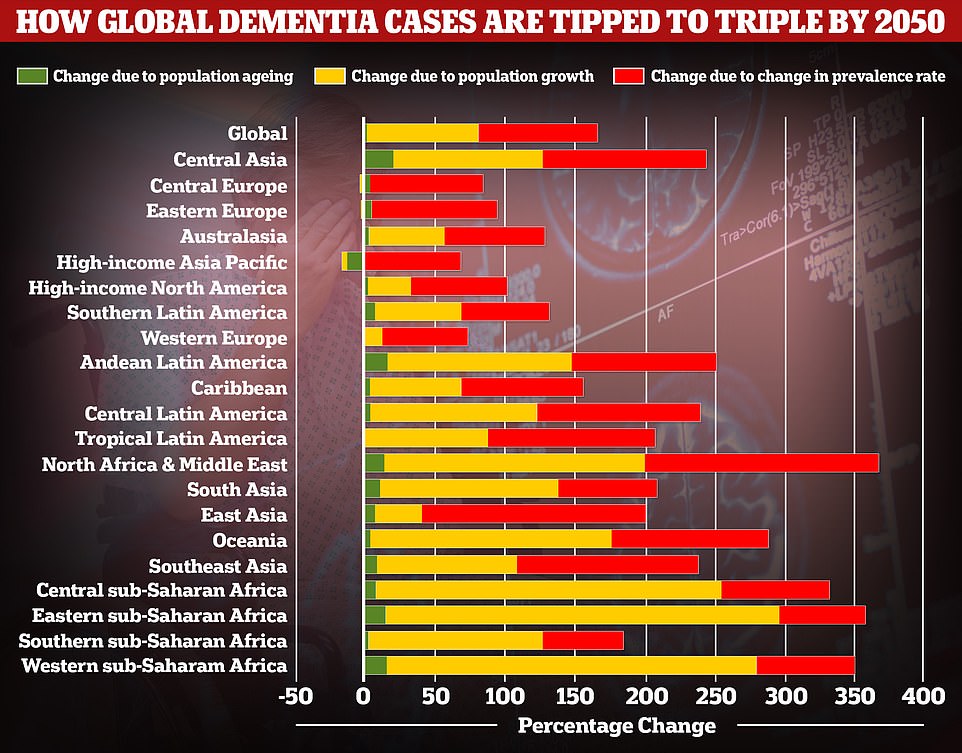





A study by researchers at the University of Washington School of Medicine revealed that global dementia cases are set to nearly triple by 2050, from 57.4million to 152.8. But the rate of illness is expected to increase varies between different parts of the world. In Western Europe, cases are expected to rise by just 75 per cent, mainly due to an ageing population, while they are expected to double in North America. But the biggest increase is expected to be seen in North Africa and the Middle East, where cases are projected to rise by 375 per cent
Alzheimer’s ‘vaccines’ to prevent the disease from ever starting
In November, scientists at Brigham and Women’s Hospital in Boston in the US started a phase one trial of nasal spray that is hoped could be a viable vaccine for Alzheimer’s.
The spray uses the chemical Protollin to stimulate a person’s immune system.
It’s thought to activate white blood cells that can clear plaques of amyloid beta protein that build up around brain cells and cause them die in Alzheimer’s patients.
The vaccine is given as a two-dose regimen, administered one week apart. The early trial involves 16 participants between the ages of 60 and 85.
All participants displayed early symptoms of Alzheimer’s disease, like mild cognitive impairment and early signs of plaque build-up in the brain, but have not been diagnosed with Alzheimer’s yet.
The trial will hope to find whether or not the vaccine is safe, and what dosage doctors should use for it.
The treatment will need to go through two more rounds of trials to see if it actually works, which is generally where most medicines fail in the development cycle.
This means it could be years away from being rolled out as a general treatment.
The potential vaccine is the result of 20 years of research from Dr Howard Weiner, the co-director of the Ann Romney Center for Neurologic Diseases at the US hospital.
‘The launch of the first human trial of a nasal vaccine for Alzheimer’s is a remarkable milestone,’ he said at the time.
‘If clinical trials in humans show that the vaccine is safe and effective, this could represent a nontoxic treatment for people with Alzheimer’s, and it could also be given early to help prevent Alzheimer’s in people at risk.’
Antibody treatments attempting to stop Alzheimer’s in its tracks
A number of potential Alzheimer’s drugs have aimed to use specially developed antibodies, part of the body’s immune system, to help clear plaque from the brain.
Most of these have failed when trialled on humans but that hasn’t stopped scientists from developing new ones in an attempt halt or even reverse the spread of the disease.
One of the most promising is TAP01, which was developed by a team of researchers from the University of Leicester, the University Medical Center Göttingen in Germany and British medical research charity LifeArc.
TAP01 works by targeting the amyloid beta protein, while it is in a soluble form rather than other Alzheimer’s antibody candidates which attempt to target the protein after it becomes the plaque in the brain.
Professor Thomas Bayer, from Göttingen, said: ‘We identified an antibody in mice that would neutralise the truncated forms of soluble amyloid beta, but would not bind either to normal forms of the protein or to the plaques.’
The researchers want to artificially create versions of this antibody and ‘humanise’ it — so the immune system wouldn’t reject it.
Further tests on rodents with Alzheimer’s using these antibodies produced promising results.
Rodents showed improvements to brain activity and memory, as well as reduced levels of the protein in the brain.
The authors of the study hope TAP01 could act as either therapeutic treatment for Alzheimer’s patients or as a kind of ‘vaccine’ preventing the disease from developing in the first place.
Leicester’s Professor Mark Carr, an expert in chemical biology, added: ‘While the science is currently still at an early stage, if these results were to be replicated in human clinical trials, then it could be transformative.’
‘It opens up the possibility to not only treat Alzheimer’s once symptoms are detected, but also to potentially vaccinate against the disease before symptoms appear.’
The researchers are now looking to find a commercial partner to take TAP01 through clinical trials in humans.
Another Alzheimer’s antibody treatment currently being trialled in the US is gantenerumab.
It is supposed to work in the more conventional way, by clearing amyloid plaque once it has already built-up in the brain.






Antibody treatments which have been used for years in things like cancer treatment or organ transplants, sprung to wider attention as a potential Covid treatment during the pandemic. One such product from AstraZeneca, called Evusheld, or Covid antibody therapy AZD7442 could allow people who can’t be protected by vaccines a chance to return to normal life
In the latest trial, completed in June last year, 144 people with a genetic risk of Alzheimer’s were either given gantenerumab or a placebo for up to seven years. But neither drug prevented or slowed cognitive decline in the participants.
The drug reduced plaque in their brains but participants were not found to be any less forgetful, and there was no statistical improvement to their cognition.
Experts are now setting out to test if the drug works in patients at even earlier stages of the disease, with its developers still holding out hope.
‘The drug’s ability to shift multiple Alzheimer’s biomarkers toward normal indicates that it is positively affecting the disease process,’ the researchers said.
In March this year, a new gantenerumab trial, called SKYLINE was launched and will run for four years.
It will recruit 1,200 participants between the ages of 60 and 80 who show signs of protein plaque building up in their brains but are yet to suffer any cognitive decline.
Participants will receive a weekly, or biweekly dose of gantenerumab and their outcome will be compared to a placebo group.
Dr Dallas, from the University of Reading, told MailOnline this was still the best area for potential new ways to combat the disease.
‘We are further down the line with antibodies and vaccines to understand how these can disrupt the disease,’ he said.
‘The Alzheimer’s drug pipeline is filled with over 30 vaccines and antibodies that are currently being tested as active agents against Alzheimer’s disease.
‘There are more antibody therapies that are currently in Phase 3 trials, so there are likely to be some announcements in the next 12 months about their status.‘
He added that he expects new Alzheimer’s medicines will become available in the next five to 10 years which will provide great benefit to patients, but these would not be a cure.
Professor David Smith, an expert in pharmacology at the University of Oxford, said it was important to note all current antibodies and vaccines being trialled are based on the hypothesis the amyloid beta protein is behind Alzheimer’s.
The fact these medications have so far failed to work against Alzheimer’s meant we could need to explore more about what causes the disease, he added.
‘Antibodies and vaccines are all designed on the amyloid hypothesis, which has not yet been proven,’ he said.
‘Indeed, nearly all trials have failed and this calls into question the validity of the hypothesis.’
Antibody treatments, which have been used for years in things like cancer treatment or organ transplants, sprung to wider attention as a potential Covid treatment during the pandemic.
An antibody cocktail called Evusheld was made by Covid jab maker AstraZeneca and approved for use in the UK last year for immunosuppressed patients who might not respond well to vaccines.
The brain zapping helmet that could help reverse Alzheimer’s
It sounds like something out of science fiction. But some scientists think that zapping the brain with infrared light could help reverse Alzheimer’s.
In October last year, researchers from Durham University published results from a trial where participants wore a helmet that shot pulses of infrared light, which is invisible to the naked eye, directly into the brain.
This infrared light burst stimulates brain cells, providing them an energy boost and boosting blood flow, process called ‘photo biomodulation’.
In theory, this should stimulate the brain to activate immune cells that sweep away the toxic proteins linked to dementia.
Researchers also believe the therapy increases levels of nitric oxide, and therefore improves blood flow in the brain, making sure more oxygen can reach brain cells.
The £7,250 helmet, which has 14 in-built lights, was trialled on 14 healthy Britons who wore it twice per day in six minute long sessions for a month.
Participants underwent memory, verbal and motor skill tests before and after their month-long treatment which were then compared.
Their results were also compared to 13 other Britons who wore a dummy helmet for the same period and acted as a placebo.
Scientists found participants who wore the real helmet had a significant improvement on their test scores compared to the placebo group.
The helmet was developed by Dr Gordon Dougal, a GP in County Durham, who worked with Durham University’s Dr Paul Chazot, an expert in biosciences.
Dr Dougal said the helmet ‘may well help dying brain cells regenerate into functioning units once again’. He added, however: ‘Much more research is needed to fully understand the mechanism of action.’
‘We’ve shown what appears to be real improvements in memory and other neurological processes for healthy people when their brains are exposed to a specific wavelength of infrared light for consistent, short periods of time.
‘This could provide a novel disease-modifying strategy for dementia, with the potential to alleviate many of the serious problems faced by people with dementia and reduce the burden on their caregivers.’
The British duo also trialled the technology on 56 dementia patients in the US, 39 of which received the treatment and the remainder acting as a control.
Working alongside Texan academics, they found people who used the helmet scored up to 20 per cent better on mental exams after an eight-week cycle of treatment.
This compared to a 6.5 per cent improvement in scores in the control group. Further research on the technology is said to be exploring if it could help reverse Alzheimer’s.
Dr Dallas added that the evidence so far if using light was an effective Alzheimer’s it would not be a ‘quick fix’ and people would likely need continual therapy to keep their brains healthy.
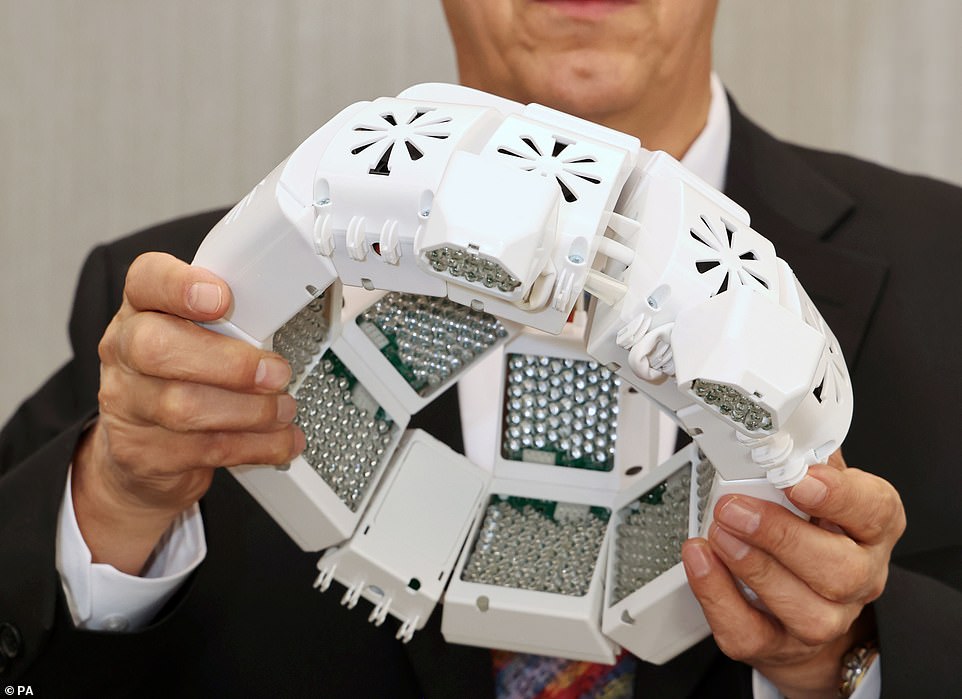





The study saw 14 healthy Britons wear the helmet (pictured) for six minutes twice a day for one month, with infrared light was beamed into their brains at a wavelength of 1068 nanometres






Lead researcher Dr Paul Chazot (left), who has spent 20 years of studying particular infrared wavelengths for dementia treatment, pictured with GP Dr Gordon Dougal, who devised the helmet and Tracy Sloan who used it to improve her memory
Putting patients in oxygen chambers
Giving Alzheimer’s patients oxygen therapy by placing them in pressurised rooms could provide a potential non-pharmacological cure for Alzheimer’s.
In November, Israeli researchers published the results from a study of six patients with mild cognitive impairment, considered a pre-cursor to dementia.
Their symptoms improved after having five 90-minute oxygen treatments a week for three months, scientists found.
Called hyperbaric oxygen therapy (HBOT) – the treatment involves patients inhaling oxygen through a mask in a pressurised chamber.
It sometimes used by athletes to help them recover quicker and celebrities who claim it beats stress.
The treatment significantly increases the amount of oxygen in the body, which advocates say encourages tissue to heal – including in the brain.
When the treatment has been administered to mice, it removed amyloid plaques from the brain. Experts believe the therapy works by changing the structure of vessels in the brain and increasing blood flow.
Reduced blood flow to the brain has already been linked with the onset of dementia.
Dr Dallas said it was too early to say if it would help treat Alzheimer’s in humans.
‘In relation to Alzheimer’s only six participants were reported in this study which limits the conclusions that can be made,’ he said.
He also added that it could be impractical to use on a mass scale.
‘Another factor to consider is that the intervention consisted of 60-to-90-minute sessions a day which is asking a lot for people living with dementia to commit to,’ he said.
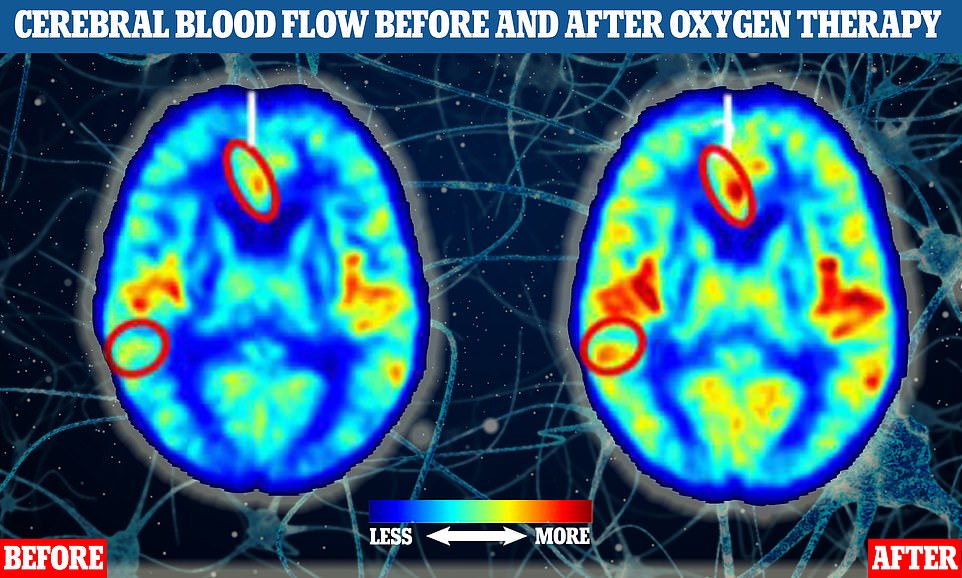





The two brain MRI scans of human participants indicate blood flow before (left) and after (right) one of the study participants had oxygen therapy. Areas where there are more yellow, orange and red tones indicate higher levels of blood flow. The two circled parts of the brain show where blood flow increased significantly in response to the treatment
Stem cells to reverse the damage Alzheimer’s causes
Stem cells are ‘building block’ cells that can develop into many different cell types, including brain or nerve cells.
Some scientists hope they have to potential to repair brain damage caused by neurological conditions, such as dementia.
One year-long trial, completed in September last year, saw 33 people with Alzheimer’s treated with a high or low does of stem cells derived from bone marrow donations from healthy individuals, or a placebo.
Made by Longeveron, a cell-based therapies company in Florida, the treatment is called Lomecel-B and was delivered as a single dose via an intravenous infusion.
The hope was that the stem cells would improve blood vessel health in the brain as well as reduce inflammation helping to countereffect the damage caused by Alzheimer’s.
Results from the trial were published in March and found patients who had the treatment showed a slower decline in their cognitive abilities than the placebo group, with data suggesting Lomecel-B helped increase blood vessel function and reduced brain inflammation as hoped.
Inflammation is considered a key contributor to the development of Alzheimer’s.
Brain scans of the patients three months after treatment also showed those on a high Lomecel-B dose saw an increase in the size of the left hippocampus, the section of the brain critical for memory formation which is damaged as Alzheimer’s disease progresses.
Dr Anthony Oliva, the company’s senior scientist, said they were encouraged by the results.
‘With a single dose of Lomecel-B, we observed several lines of preliminary evidence supporting multiple mechanisms of action of Lomecel-B, and most importantly, the data supports the potential of Lomecel-B as disease modifying for Alzheimer’s,’ he said.
The most recent trial, which was phase one, was primarily designed to ensure Lomecel-B was safe for use in people and no safety concerns were found.
A new larger scale trial, phase two, is now underway having started in December last year. This trial is designed to test if single dose or multiple doses of Lomecel-B are effective for treating mild Alzheimer’s disease.
Using gut bacteria
Some experts think targeting the gut, not the brain, could be the key to beating Alzheimer’s.
The theory is based on the role of the microbiome, a community of microorganisms that live in your digestive tract, in wider health.
Previous studies have suggested gut bacteria could be involved with a variety of brain functions, from appetite control to conditions like depression, anxiety — and now dementia.
In March this year, experts unveiled multiple studies highlighting the potential interaction of the microbiome and Alzheimer’s.
One revealed how the microbiomes of patients with the condition can differ massively to those without the disorder.
Another found rodents given faecal transplants directly from rats with Alzheimer’s perform worse on memory tests.
And there are trials under way testing some drug’s ability to recondition gut bacteria to improve brain health.
In theory, the patients’ gut bacteria influence the levels of inflammation in the body which then impacts the brain via the blood supply.
Experts hope that Alzheimer’s treatments could be developed to target the gut, which could then improve the condition in the brain.
Dr Edina Silajdžić, a neuroscientist from King’s College London, said: ‘Most people are surprised that their gut bacteria could have any bearing on the health of their brain.
‘But the evidence is mounting — and we are building an understanding of how this comes about.
‘Our gut bacteria can influence the level of inflammation in our bodies, and we know inflammation is a key contributor to Alzheimer’s.’
She was behind research that compared the microbiomes of 68 people with Alzheimer’s and a similar number who did not.
Blood and stool samples were taken from all of the participants and analysed at a biological lab in Italy.
These tests revealed that people with Alzheimer’s had a distinct microbiome as well as more inflammation markers.
Follow-up experiments involving treating brain stem cells with the blood from people with Alzheimer’s.
These were found to be less able to grow new nerve cells than controls treated with blood from people without the disease.
Dr Silajdžić said: ‘This leads us to believe inflammation associated with gut bacteria can affect the brain via the blood.’
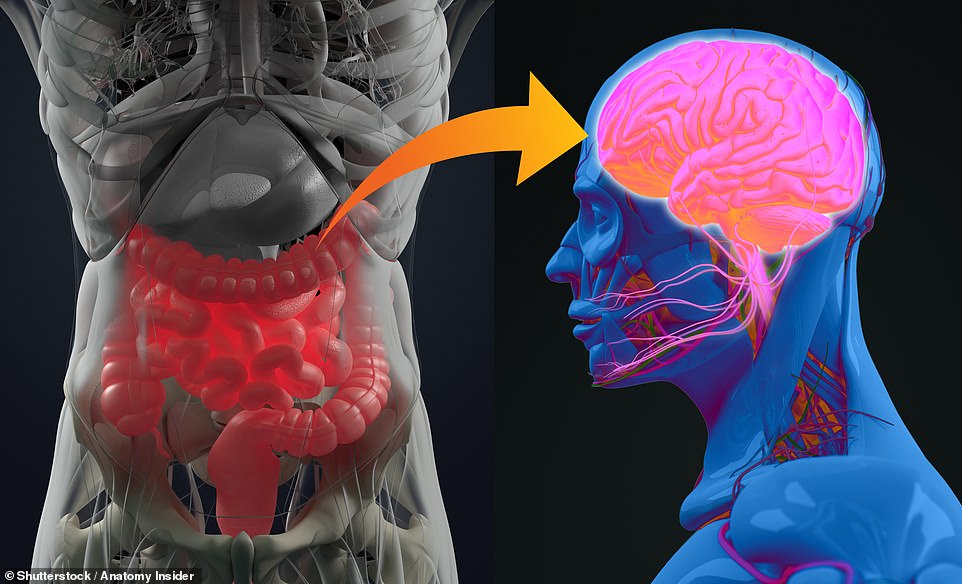





UK researchers have presented the results of two experiments potentially linking the microbiome of the gut to the brain
Professor Nolan said the gut could present an alternative and easier part of the body to target for potential Alzheimer’s treatments.
‘While it is currently proving difficult to directly tackle Alzheimer’s processes in the brain, the gut potentially represents an alternative target that may be easier to influence with drugs or diet changes,’ she said.
Alzheimer’s Research UK’s director of research, Dr Susan Kohlhaas, said the early studies provided a good base for further work on the relationship between gut bacteria and Alzheimer’s.
‘Taking these results together reveals differences in the makeup of gut bacteria between people with and without dementia and suggest that the microbiome may be driving changes linked to Alzheimer’s disease,’ she said.
‘Future research will need to build on these findings so that we can understand how gut health fits in to the wider picture of genetic and lifestyle factors that impact a person’s dementia risk.’
Dr Dallas said gut bacteria may prove useful in detecting those at risk from dementia, but science had yet to find ways to use the microbiome to protect brain health.
However he added scientists were keeping a close eye on a trial in China of a drug called GV-971, which is derived from brown algae.
GV-971 reportedly reconditions the gut microbiome to reduce inflammation in the brain, though Dr Dallas said more details were needed on how exactly it works.
‘Debate around how this drug works is ongoing but larger global trials are set to be undertaken to provide the evidence that is currently lacking for this specific drug,’ he said.
Professor Smith added while the microbiome and its relation to Alzheimer’s was ‘worth exploring further’ it was ‘too early’ to say if it was a valid approach.
Source: | This article originally belongs to Dailymail.co.uk