A team of international researchers including those from the University of Adelaide have taken a well-known chemical reaction as the basis of a new generation of targeted pain relief medication.
The local team, including Dr. Thomas Avery and Ph.D. candidate Dion Turner and led by Professor Andrew Abell of the Department of Chemistry and the ARC Center of Excellence for Nanoscale BioPhotonics (CNBP), Institute for Photonics and Advanced Sensing (IPAS) at the University, alongside Associate Professor Peter Grace from the University of Texas MD Anderson Cancer Center, published their findings in Nature Biotechnology.
Researchers from Texas Southern University were also involved in the research.
“Our team has created a targeted prodrug (a compound which metabolizes inside the body into a pharmacologically active drug), and found it to be capable of relieving chronic pain during preclinical trials,” said Professor Abell.
“We believe we were the first people to come up with the idea of using this particular chemical reaction in a biological sense, and we already see potential for its use in other settings.”
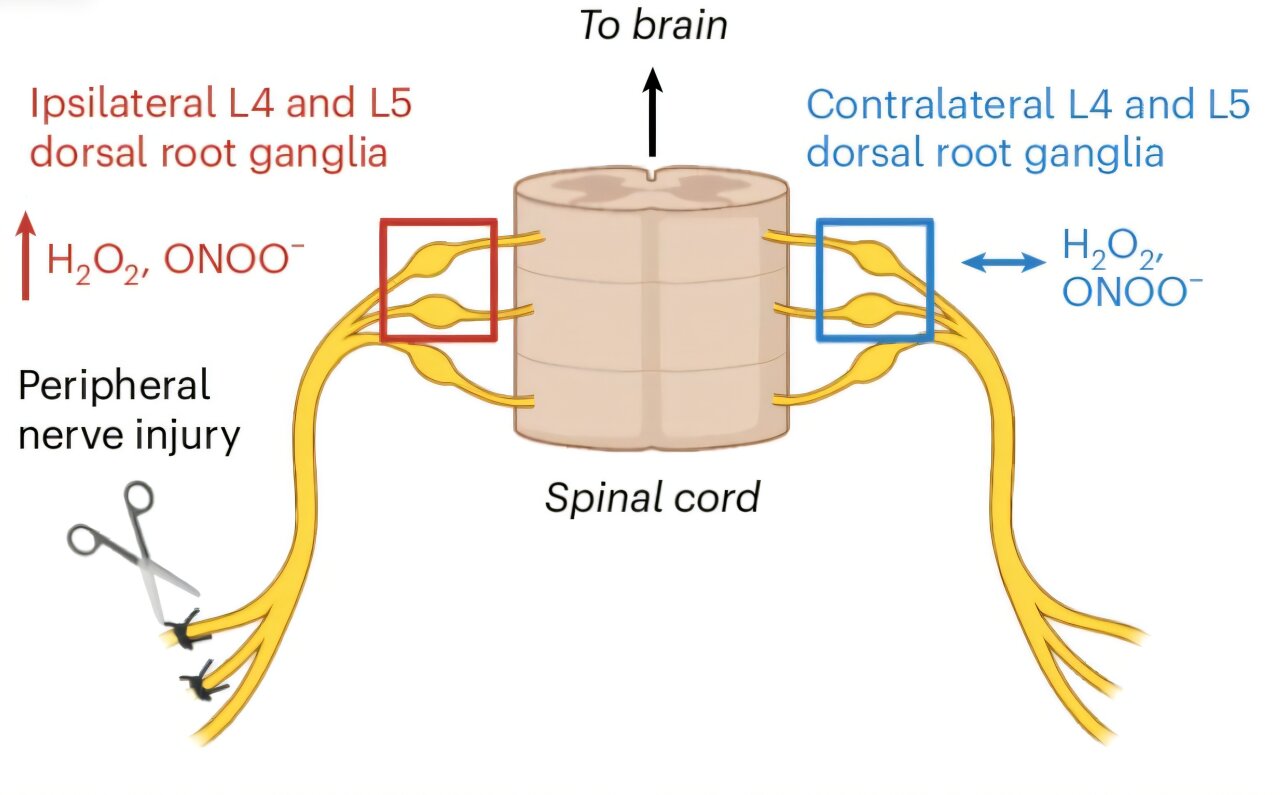
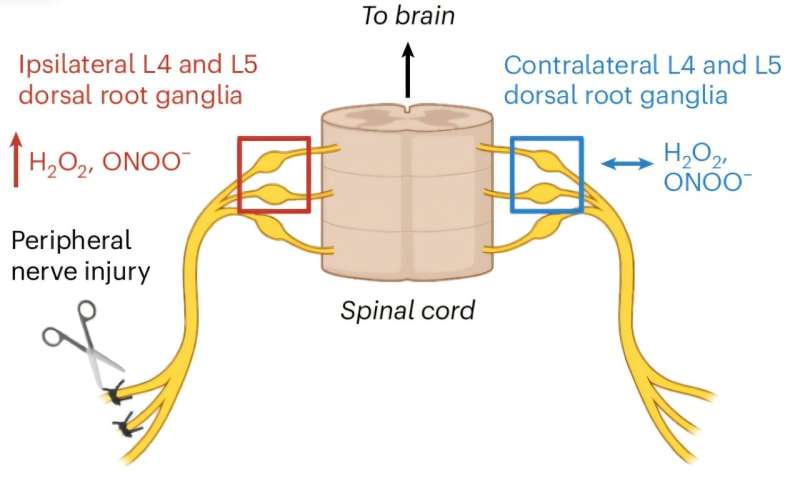
The mechanism of action for the targeted prodrug involves activation of the active drug by a chemical reaction with reactive oxygen species such as hydrogen peroxide, which are present in much higher amounts at sites of pain than the rest of the body.
This means that the prodrug, designed and made in Professor Abell’s laboratory, is distributed around the body as an inactive chemical until it reaches a site of pain where it is then converted into the active drug.
The prodrug was tested in both chemical and preclinical models and found to provide localized relief of sciatic nerve injuries, as well as other models of chronic pain featuring oxidative stress like osteoarthritis, chemotherapy-induced peripheral neuropathy and diabetic neuropathy.

Testing found multi-day oral administration of the compound six months after the injury reversed hypersensitivity to touch and cold stimuli; while further tests demonstrated the effects of the drug were dose dependent, with maintained pain relief upon repeated dosing.
“This showed us that the compound did not induce a tolerance, which is a major limiting factor to powerful painkillers like morphine,” said Mr. Turner.
“Chronic pain remains a large unmet medical need and nonaddictive treatments like this would revolutionize the field, which is currently dominated by addictive opioids.”
The project will now undergo more pre-clinical trials to determine effectiveness and safety.
“The few drugs available to treat chronic pain are only effective for about one in six people, and they simply reduce the activity of nerves that send pain signals,” said Associate Professor Grace.
“Our new prodrug addresses an underlying problem by reducing the molecules that are responsible for sending the pain signals. This has potential for a new approach to chronic pain treatment.”
Members of the team have also been involved in the formation of a company, Immunologic, which is currently raising funds to support the completion of the pre-clinical phase work and the development of this prodrug for human clinical trials.
“This project is capitalizing on the promise of resolving oxidative stress and inflammation to effectively treat pain,” said Immunologic Executive Chairman and CEO Stephen Collins MD Ph.D.
“There are no other companies doing something quite like this.”
More information:
Thomas D. Avery et al, Site-specific drug release of monomethyl fumarate to treat oxidative stress disorders, Nature Biotechnology (2024). DOI: 10.1038/s41587-024-02460-4
Provided by
University of Adelaide
Citation:
Researchers discover localized pain relief using known chemical reaction (2024, November 5)
retrieved 5 November 2024
from https://phys.org/news/2024-11-localized-pain-relief-chemical-reaction.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.