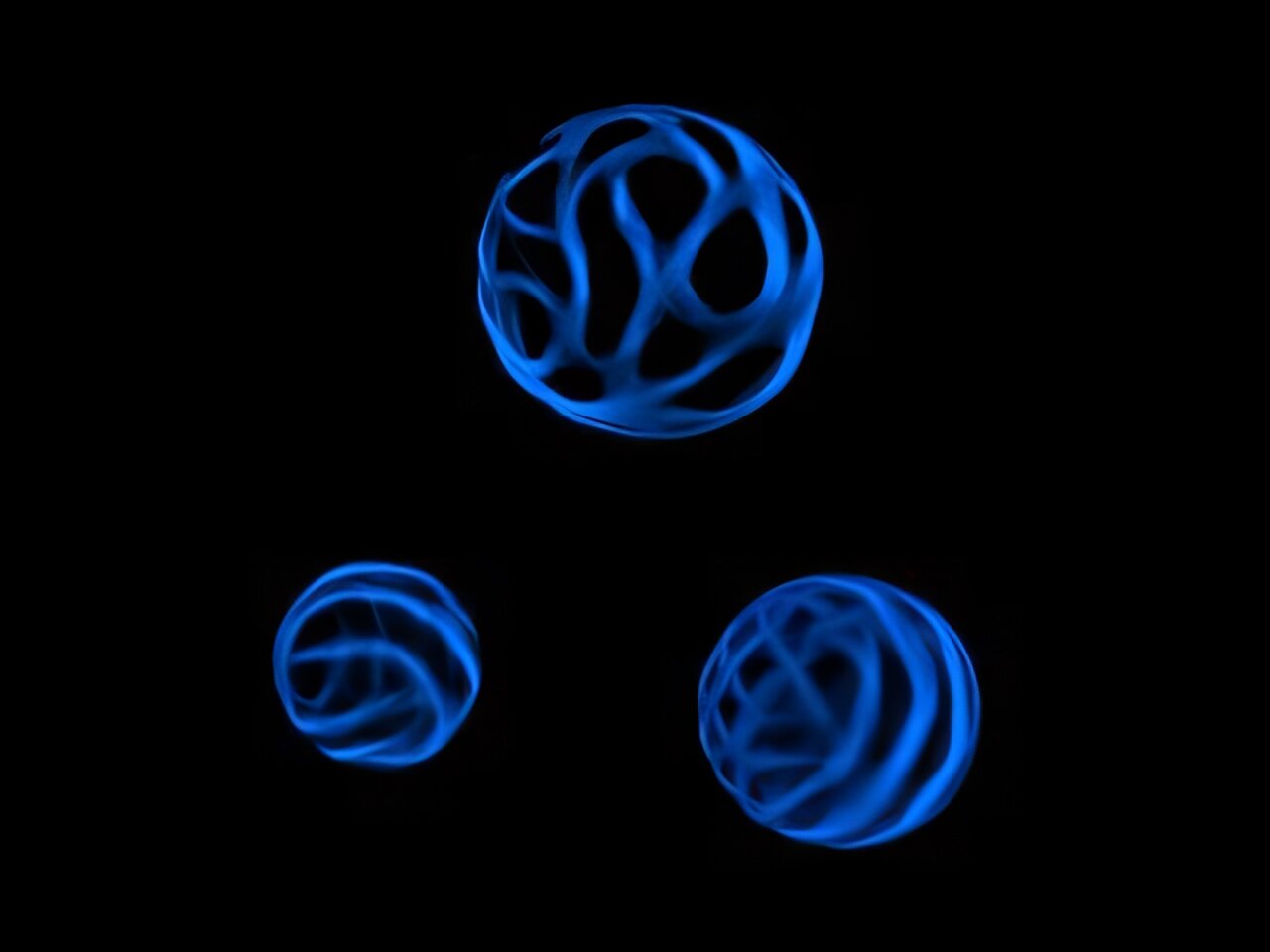
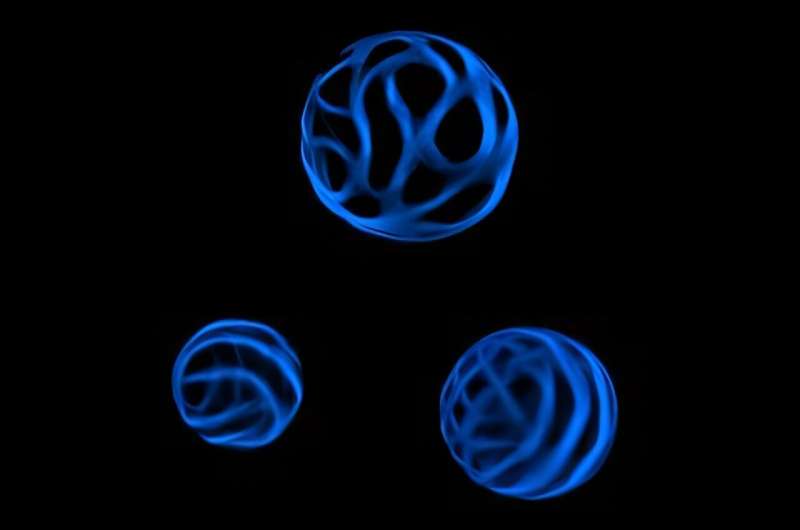
A new method developed by Penn State biologists allows them to turn stripped-down plant cells into other types of cells, similar to the way stem cells differentiate into different cell types. Using this method, the research team explored the banding patterns that increase the stability of plant cell walls—much like the corrugated patterns in cardboard—and how they are created. Additionally, the researchers revealed how the assembly of these structures can go astray in different mutant plant cells, which they said could ultimately inform methods to break down plant cells for biofuels.
A paper describing the research appeared in the October issue of the journal The Plant Cell.
Cellulose, a structural component of plant cell walls, is an abundant and promising source of biofuels. However, common techniques to extract cellulose from cell walls, which involve removing other entangled large molecules called polymers, require chemical solvents, enzymes and reactions at high temperatures, which add cost and complexity to the process. Improving the understanding of how cell walls are built could illuminate new, more cost-efficient ways to extract cellulose, according to the researchers.
“In recent years, researchers have explored a variety of ways to potentially improve the efficiency of the cellulose extraction process, for example by manipulating other polymers in the cell wall that can get in the way, like xylan and lignin,” said Sarah Pfaff, postdoctoral scholar at the Penn State Eberly College of Science, who led the research.
“But the unique structures formed by ‘xylem tracheary element’ cells often fail to develop properly in these mutant plants, which causes the cells to collapse and ultimately reduces plant growth and the amount of extractable cellulose. In this study, we explore how these unique cell walls are assembled in healthy plant cells and also how this process goes wrong in mutants.”
Xylem tracheary elements (XTEs) are a type of cell that allow water to move from a plant’s roots to its leaves, and they have remarkably thick cell walls. Unlike in other cells, Pfaff said, polymers like cellulose, xylan and lignin are deposited in specific locations in the cell walls of XTEs, creating a banding pattern. When these patterns are not formed properly in mutant cells, the cells can collapse from the pressure of moving water against gravity.
“The banding patterns in xylem tracheary elements act a lot like the corrugated pattern in cardboard, adding stability to the cell wall,” Pfaff said. “Using traditional methods, it was difficult to see individual cells to understand how this banding pattern breaks down in mutant cells. So we developed a method that lets us observe individual cells without any of the neighboring cells getting in the way.”
The new method takes advantage of protoplasts, individual cells that have been stripped of their cell walls, which the researchers provide with nutrients and what Pfaff calls a “genetic trigger” to differentiate into a new type of cell. Although protoplasts have been used in a variety of previous plant studies, the new method allows the researchers to observe the cells as they differentiate into the unique XTE cell type.
“We provide protoplasts with a transcription factor—a sort of genetic trigger—so that they develop into a new cell type based on that cue,” Pfaff said. “It’s a bit like stem cells in that we can reprogram their developmental fate and watch them turn into entirely different cell types. In this study, we specifically induced protoplasts from both healthy and mutant plants to turn into xylem tracheary elements and observed how the banding patterns in their cell walls formed.”

The researchers found that certain interactions between cellulose and xylan are necessary for the bands to form correctly and that a properly assembled cell wall network of polymers acts as a scaffold to dictate the banding pattern. They also found that in different mutant cells, the banding pattern failed in different ways.
“Previous research has focused on how the inside of the cell might impact the cell wall, which is synthesized outside of the cell, but we found that it also works in the other direction,” Pfaff said. “The cell wall structure can also impact what’s happening inside the cell, and they can feed back on each other. This work provides important insights into how cell walls are created and how these kinds of mutants might be viable in the future.”
According to Pfaff, understanding how cell walls are built is of interest in forestry, materials science, as well as for biofuel production. The research team plans to use their new method to explore how other types of cell walls are created.
“Instead of breeding mutant plants together to get multiple different genetic traits in one plant, which might take many months, now you can explore different combinations in individual cells,” Pfaff said. “You could also use different kinds of genetic triggers to study other cell types, which could have implications across plant biology.”
In addition to Pfaff, the research team at Penn State includes Edward Wagner, senior research technician, and Daniel Cosgrove, Eberly Family Chair of Biology.
More information:
Sarah A Pfaff et al, The structure and interaction of polymers affects secondary cell wall banding patterns in Arabidopsis, The Plant Cell (2024). DOI: 10.1093/plcell/koae233
Provided by
Pennsylvania State University
Citation:
Stem cell-like approach in plants sheds light on specialized cell wall formation (2024, October 31)
retrieved 1 November 2024
from https://phys.org/news/2024-10-stem-cell-approach-specialized-wall.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.