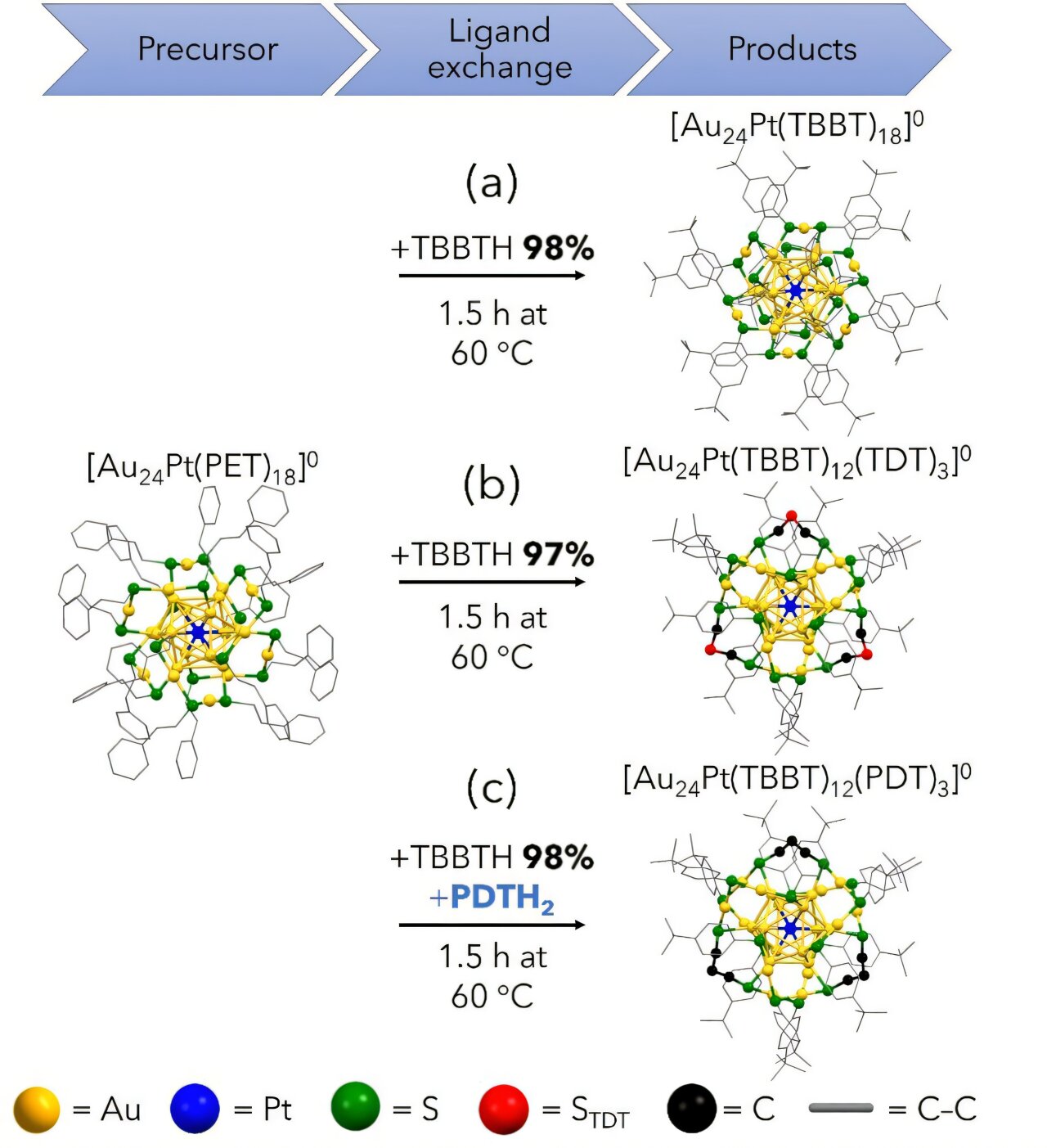
![Synthetic conditions and geometric structures of (a) [Au24Pt(TBBT)18]0, (b) [Au24Pt(TBBT)12(TDT)3]0 and (c) [Au24Pt(TBBT)12(PDT)3]0. Credit: Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.4c10868 Improvement of catalytic activity by controlling the surface structure of metal particles](https://skynews.icu/wp-content/uploads/2024/10/Synthesis-method-improves-catalytic-activity-by-controlling-the-surface-structure.jpg)
Fine-tuning the reactions that produce hydrogen (H2) for use as a clean fuel is an important endeavor to fight against climate change. Researchers at Tohoku University and the Tokyo University of Science have succeeded in improving the catalytic activity for this reaction. The researchers established a synthesis method that can control the surface structure of small metal particles with a diameter of about 1 nm.
The results were published in the Journal of the American Chemical Societyon October 15, 2024.
Hydrogen is an environmentally-friendly alternative to other energy sources such as fossil fuels. One method to extract the hydrogen from water is called the hydrogen evolution reaction (HER): a reaction driven by electricity that typically uses an electrocatalyst to lower the activation energy (and thus make the reaction more efficient). However, one downside is that it currently requires expensive rare metals such as platinum.
In order to make hydrogen fuels a more viable, affordable option, researchers looked at cheaper metals. They created an aggregate metal nanocluster (NC) combining gold (Au) with platinum (Pt) to achieve this. Since gold is not nearly as rare or costly as platinum, this combination lowers the price of producing hydrogen.
This study looked at AuPt nanoclusters (NCs) with two novel geometrical and electronic structures. “Compared to the conventional AuPt NCs, these structures had less bulky ligands, which was expected to facilitate access to the active site and improve catalytic activity,” said Professor Yuichi Negishi (Tohoku University).
“We’ve been analyzing how to improve these reactions for a while now, so we got a hint from our previous study where the length of the ligand had a significant effect on HER activity.”
Upon testing their newly-generated AuPt NCs, they discovered that they achieved 3.5 and 4.9 times higher catalytic activity for the HER compared to conventional AuPt alloy catalysts.
This research has demonstrated a method to precisely control the surface structure of ultra-fine metal aggregates. It may also have alternative catalytic applications such as carbon dioxide reduction, carbon monoxide oxidation, alcohol oxidation, and oxygen reduction reactions.
This finding may promote the development of new functional materials and move us closer to a world where we fill up with clean hydrogen instead of gasoline.
More information:
Miyu Sera et al, Atomically Precise Au24Pt(thiolate)12(dithiolate)3 Nanoclusters with Excellent Electrocatalytic Hydrogen Evolution Reactivity, Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.4c10868
Provided by
Tohoku University
Citation:
Synthesis method improves catalytic activity by controlling the surface structure of metal particles (2024, October 25)
retrieved 25 October 2024
from https://phys.org/news/2024-10-synthesis-method-catalytic-surface-metal.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.