

Scientists at UCLA have found that the protein GPNMB, released by certain immune cells after a heart attack, plays a key role in helping the heart heal.
The protein binds to a specific receptor, encouraging tissue repair. This discovery could lead to new treatments that prevent heart failure by enhancing the heart’s ability to recover and function effectively after a heart attack.
UCLA scientists have identified the protein GPNMB as a vital factor in the heart’s healing process after a heart attack.
Their research, conducted using animal models, shows that bone marrow-derived immune cells called macrophages release GPNMB, which binds to a receptor known as GPR39. This interaction promotes tissue repair in the heart. This study offers new insights into the heart’s natural healing process and opens doors to potential treatments aimed at enhancing heart function and preventing the progression to heart failure.
In the United States, someone experiences a heart attack every 40 seconds, making it a leading cause of heart failure. Heart attacks weaken the heart and cause scarring, reducing its ability to pump blood effectively. While this scar tissue initially supports the heart’s structure, it remains permanently, overworking the remaining muscle and often leading to heart failure over time.
The Potential of GPNMB in Heart Failure Treatment
Previous clinical studies have indicated that GPNMB, or glycoprotein non-metastatic melanoma protein B, has been strongly associated with cardiovascular outcomes of individuals with heart failure. What was not clear, however, was if lacking the protein was directly responsible for the development of heart failure after a heart attack. This important distinction — whether GPNMB is just an associated biomarker or one that plays a causal role — determines if the protein can be considered a therapeutic target for future studies.
Utilizing mouse models, the researchers first established that GPNMB is not natively expressed by the heart itself but is produced by inflammatory cells originating from the bone marrow. After a heart attack, these macrophages travel to the site of injury in the heart, where they express GPNMB.
Genetic Manipulation Reveals Key Insights
The team conducted gene knockouts — inactivating the GPNMB gene — [AS1] and bone marrow transplants and observed that mice lacking the GPNMB gene exhibited dramatically worse outcomes after a heart attack, including a higher incidence of heart rupture, a fatal complication also seen in human heart failure patients. Conversely, mice with normal GPNMB expression that were given an additional dose of circulating GPNMB protein showed improved heart function and reduced scarring. Four weeks after a simulated heart attack, 67% of the animals lacking the GPNMB gene exhibited severe fibrosis, or scarring, compared with only 8% of animals in the control group.
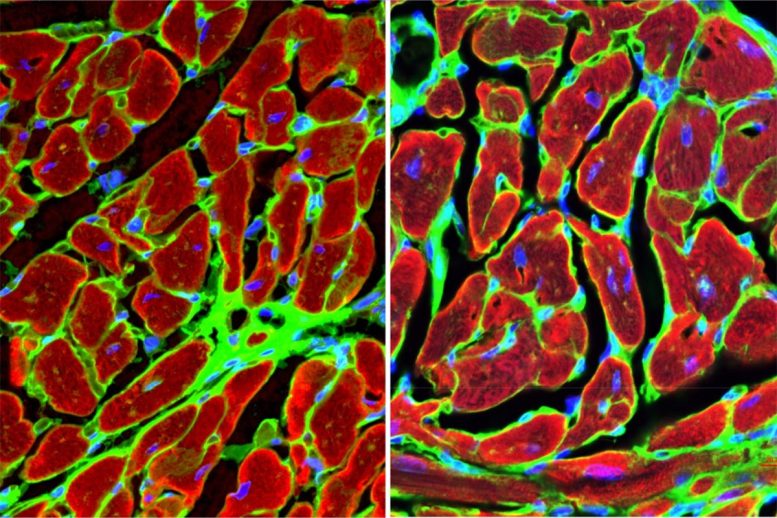
Discovering a New Pathway for Tissue Regeneration
In addition to identifying GPNMB as a signaling molecule with effects across various cell types, the researchers uncovered that it binds to GPR39, previously considered an orphan receptor, or a receptor whose binding partner is not known. This interaction triggers a cascade of signals that promote tissue regeneration and limit scarring.
Cardiovascular disease — of which heart failure is a late-stage complication — is a significant health issue, accounting for approximately one-third of all deaths worldwide. Despite its prevalence, there are no available treatments that directly enhance the heart’s ability to repair itself after a heart attack. The new study demonstrates the potential of GPNMB as a therapeutic agent, as well as GPR39 as a target, that can limit scarring, improve cardiac function and prevent heart failure.
Future Directions in Organ Repair Research
This research could also have broader implications for understanding tissue repair in other organs. As GPNMB is expressed in multiple tissues, future studies will explore its role in the repair of the brain, kidneys and other organs impacted by ischemic injury.
The study was published in Nature Cardiovascular Research.
Reference: “Bone marrow macrophage derived GPNMB protein binds to orphan receptor GPR39 and plays a critical role in cardiac repair” 25 October 2024, Nature Cardiovascular Research.
DOI: 10.1038/s44161-024-00555-4
Dr. Arjun Deb, a professor of medicine and molecular, cell and developmental biology; director of the UCLA Cardiovascular Research Theme at the David Geffen School of Medicine at UCLA; and member of the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research, led the study.
This study was supported by grants from the National Institutes of Health.
The therapeutic potential of GPNMB described in this study is under investigation and has not yet been tested in human clinical trials. The research findings are based on preclinical models, and further studies are required to assess safety and efficacy in humans.