New research reveals how our immune system uses Guanylate Binding Proteins to combat bacterial infections by forming a destructive coat around bacteria, offering potential pathways for innovative treatments.
The protein GBP1 is a crucial component of our body’s natural defense against pathogens. It combats bacteria and parasites by encasing them in a protein coat. Until recently, the mechanism behind this protective action remained a mystery. Researchers at Delft University of Technology have now deciphered how this protein functions. Their findings, published in Nature Structural & Molecular Biology, could pave the way for developing new medications and therapies, especially for individuals with compromised immune systems.
Role of GBPs in Innate Immunity
Guanylate Binding Proteins (GBPs), as biophysicist Arjen Jakobi explains, are essential to our innate immune system. “GBPs form the first line of defense against various infectious diseases caused by bacteria and parasites. Examples of such diseases include dysentery, typhoid fever caused by Salmonella bacteria, and tuberculosis. The protein also plays a significant role in the sexually transmitted infection chlamydia as well as in toxoplasmosis, which is particularly dangerous during pregnancy and for unborn children.”
GBP1: The Bacterial Coat
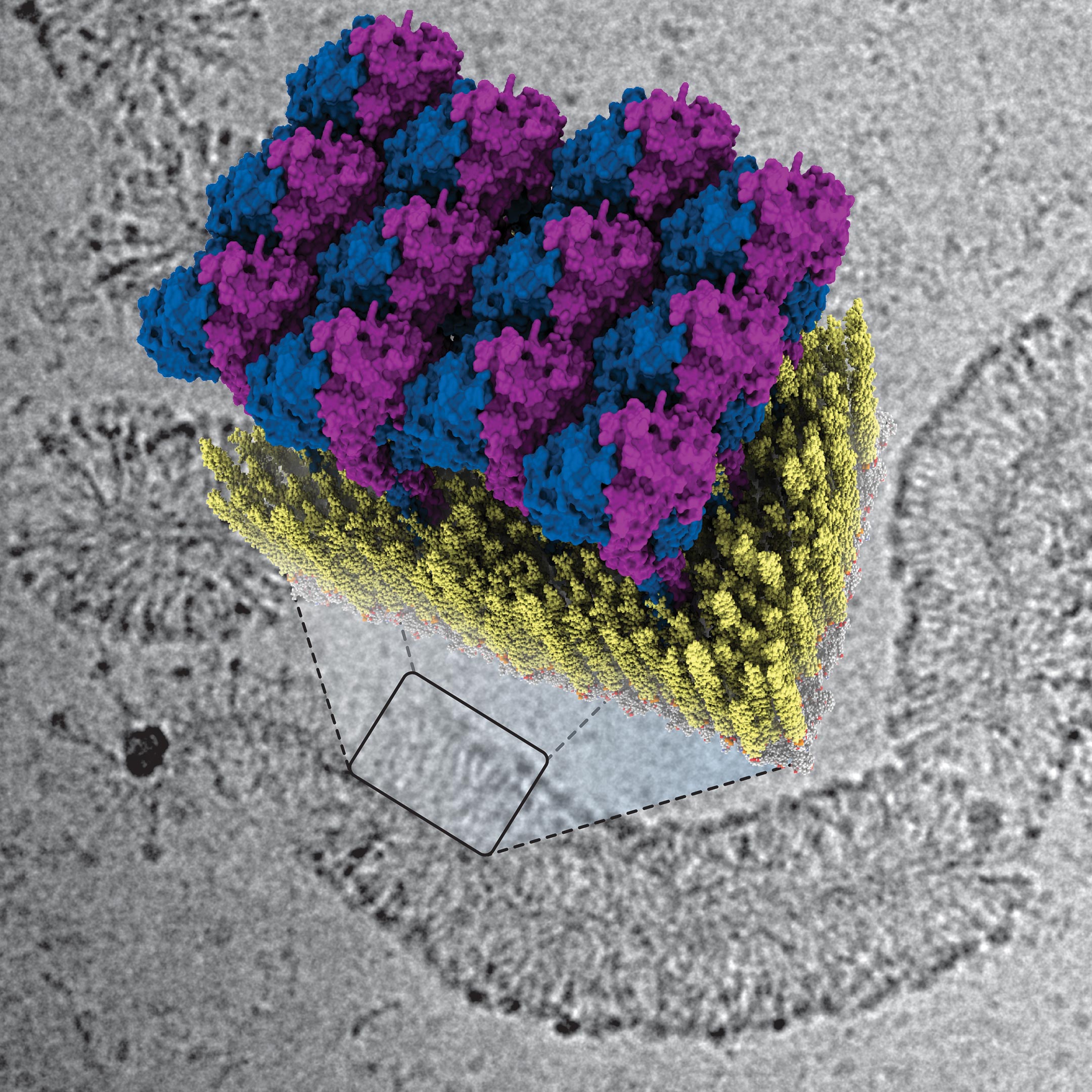
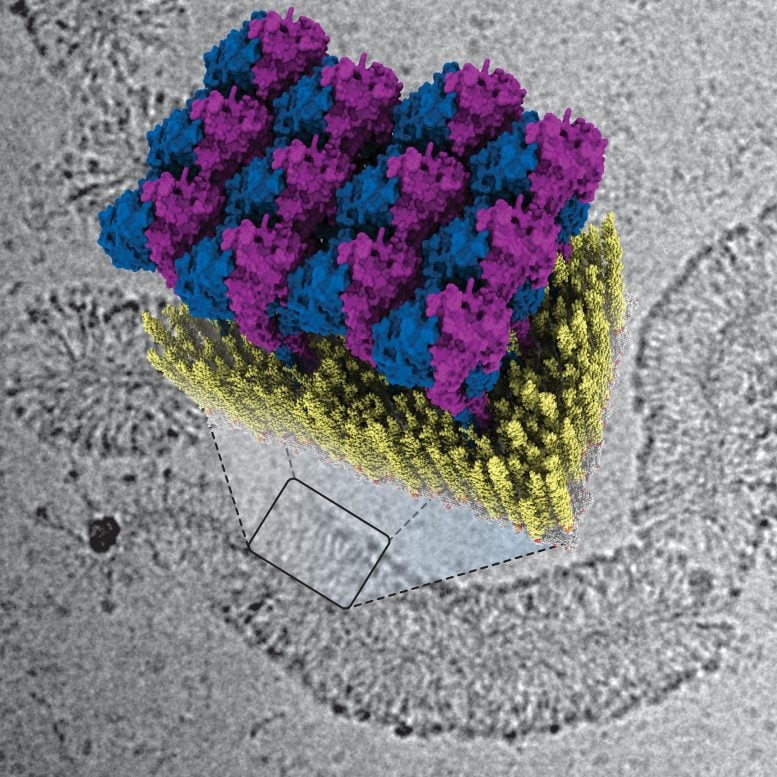
In their publication, Jakobi and his colleagues describe for the first time how the innate immune system fights against bacteria using GBP1 proteins. “The protein surrounds bacteria by forming a sort of coat around them,” explains Tanja Kuhm, PhD candidate in Jakobi’s research group and the lead author of the article. “By pulling this coat tighter, it breaks the membrane of the bacteria—the protective layer surrounding the intruder—after which immune cells can clear the infection.”
Decoding GBP’s Defensive Strategy
To decode the defense strategy of GBPs, the researchers examined how GBP1 proteins bind to bacterial membranes using a cryogenic electron microscope. This allowed them to see the process in great detail down to the scale of molecules. Jakobi: “We were able to obtain a detailed three-dimensional image of how the protein coat forms. Together with biophysical experiments conducted in Sander Tans’ research group at research institute AMOLF, which enabled us to manipulate the system precisely, we succeeded in deciphering the mechanism of the antibacterial function.”
Potential for Medicinal Applications
According to Jakobi, this research helps us understand better how our body is capable of combating bacterial infections. “If we can grasp this well, and we can specifically activate or deactivate the involved proteins through medication, it may offer opportunities to speed up getting rid of certain infections.”
Reference: “Structural basis of antimicrobial membrane coat assembly by human GBP1” by Tanja Kuhm, Clémence Taisne, Cecilia de Agrela Pinto, Luca Gross, Evdokia A. Giannopoulou, Stefan T. Huber, Els Pardon, Jan Steyaert, Sander J. Tans and Arjen J. Jakobi, 11 October 2024, Nature Structural & Molecular Biology.
DOI: 10.1038/s41594-024-01400-9