Researchers have devised a new technique to study solvation shells, providing insights into ion pair formation and electron binding energies. This discovery is key for advancing knowledge across many scientific areas.
Researchers from the Fritz Haber Institute, Sorbonne University, and Uppsala University have made a groundbreaking discovery that advances our understanding of ion behavior in solutions. Their findings were recently published in the journal Nature Communications.
Unveiling the Mysteries of Solvation Shells
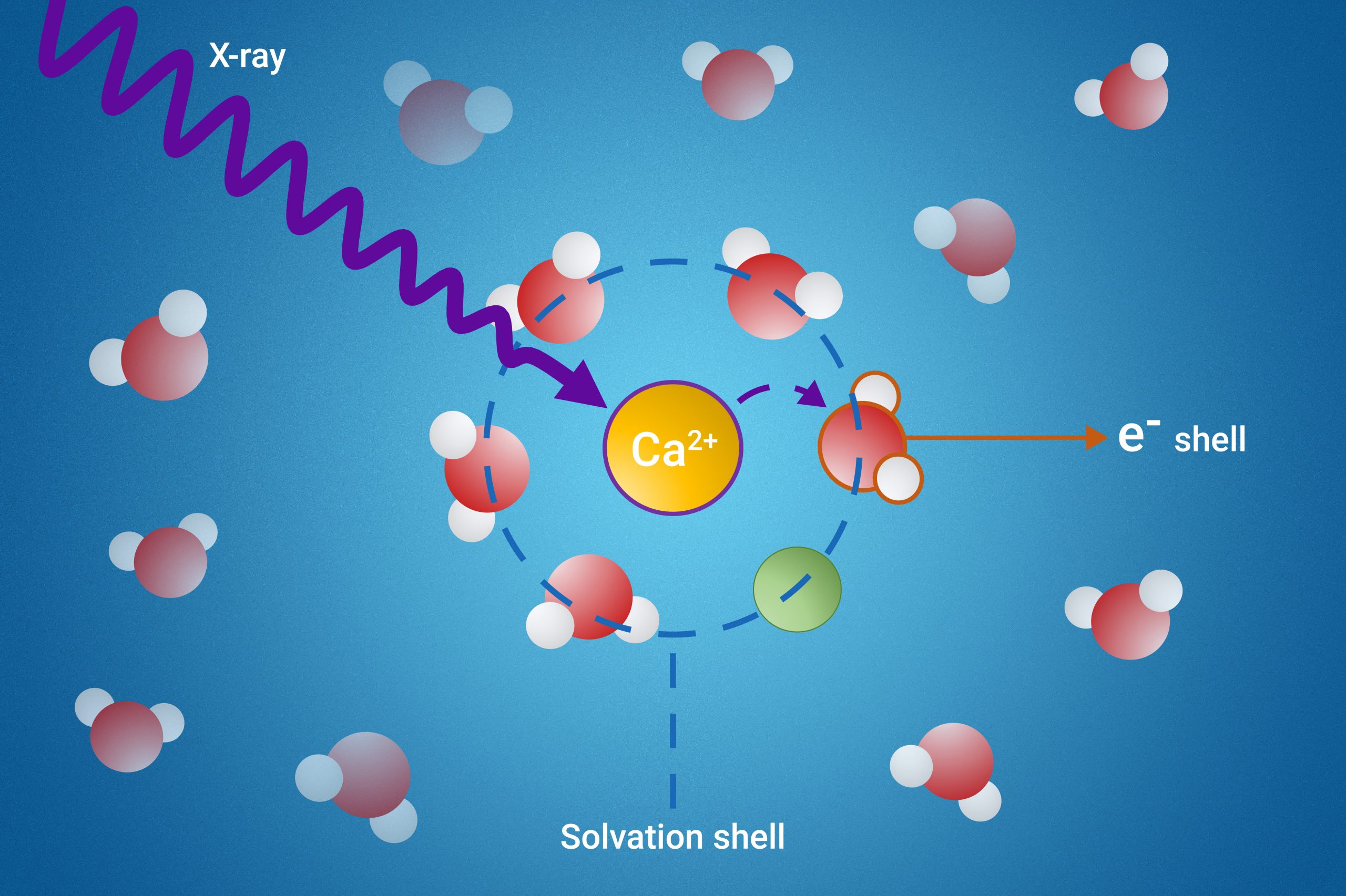
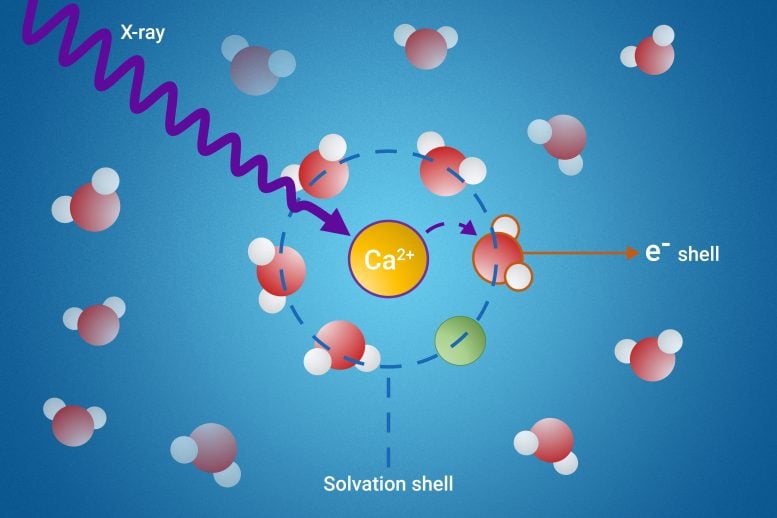
When substances dissolve in a liquid, a “solvation shell” is formed – a layer of solvent molecules that surround the dissolved particles. The solvent molecules that constitute these solvation shells can acquire wildly different properties from the other, “free” solvent molecules.
However, studying these solvation shells has always been a challenge due to their complex nature and the difficulty to target specifically the solvent molecules making up the solvation shells, but not the many other solvent molecules.
A New Window into Solvation Shells
The research team has developed a novel method to probe these elusive solvation shells using a process called resonant intermolecular Coulombic decay (ICD).
Their method involves exciting the molecules with X-rays and observing how they interact with their neighbors during the decay process. By doing so, the scientists can gain detailed insights into the properties of the solvation shell.
Key Findings
- Ion Pair Formation: The study revealed that a specific ICD process is a powerful indicator of ion pair formation. Ion pairs are pairs of charged particles that are crucial in many chemical reactions.
- Electron Binding Energies: The researchers were able to measure the electron binding energies of water molecules in the first solvation shell. This is a significant achievement, as these measurements were previously unattainable.
Why It Matters
Understanding solvation shells is essential for a wide range of scientific fields, including chemistry, biology, materials science, atmospheric science, and electrochemistry.
This new method provides a powerful tool for scientists to study these shells in greater detail, which is of relevance for these broad scientific and engineering fields.
Reference: “The solvation shell probed by resonant intermolecular Coulombic decay” by Rémi Dupuy, Tillmann Buttersack, Florian Trinter, Clemens Richter, Shirin Gholami, Olle Björneholm, Uwe Hergenhahn, Bernd Winter and Hendrik Bluhm, 13 August 2024, Nature Communications.
DOI: 10.1038/s41467-024-51417-3