

When researchers tested 111 products from major US retailers, they found unacceptably high levels of benzene at room temperature and benzene formation with heat and UV.
Research has uncovered that many benzoyl peroxide treatments for acne and rosacea become unstable and produce benzene, a carcinogen, when exposed to room temperatures and sunlight. Experts recommend refrigerating these products to reduce benzene exposure and call for more stringent testing and regulations to protect consumers.
Topical Benzoyl Peroxide Product Concerns
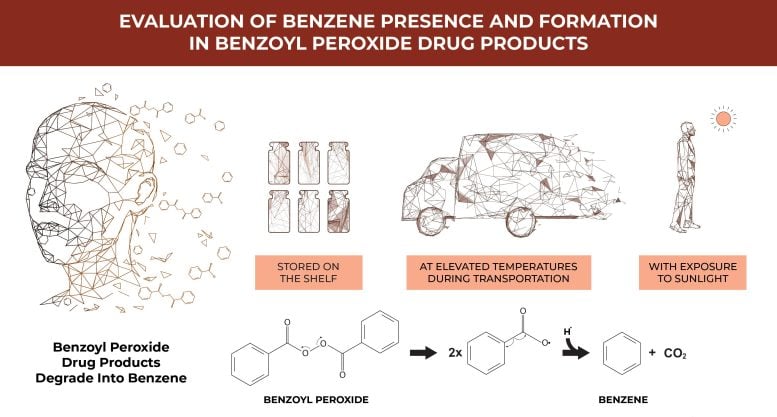
Investigators have discovered that a significant portion of topical benzoyl peroxide (BPO) acne and rosacea treatments on the market are unstable and contain dangerously high levels of benzene, a known human carcinogen. These products degrade and form benzene when stored at room temperature, at elevated temperatures, or when exposed to ultraviolet (UV) light similar to sunlight. Drug stabilization techniques such as encapsulation have proven ineffective in preventing benzene formation in BPO drug products. This critical research, detailed in the Journal of Investigative Dermatology, highlights these concerning findings.
Stability and Exposure Risks in BPO Products
The study also showed that in addition to elevated temperatures expected during use and transportation, exposure to UV light at outdoor levels is another concerning mechanism through which BPO drug products degrade into benzene, and one that appears to occur more rapidly than heated storage and transportation conditions. These products are frequently used by consumers over extended periods of time, thereby likely increasing their exposure to benzene; there is no safe level or duration of exposure to benzene.
Recommendations for Safer BPO Usage
Co-investigator Christopher G. Bunick, MD, PhD, Department of Dermatology and Program in Translational Biomedicine, Yale University School of Medicine, New Haven, CT, explains, “The potential degradation of BPO into benzene has been a topic of concern in dermatology. Our research demonstrates that BPO products can generate benzene at typical room and store shelf temperatures, while cold storage significantly reduces this formation. These findings suggest a need to recommend refrigeration of BPO products throughout the supply chain—from manufacturing to patient use—to limit benzene exposure. Until formulations are developed to prevent benzene formation, refrigeration may serve as a practical solution to minimize unnecessary exposure. Additionally, dermatologists should continue to advise patients on the appropriate use of BPO, including potential risks associated with UV exposure.”
Overview of BPO in Acne and Rosacea Treatment
BPO is a diacyl peroxide with bactericidal activity used in topical drug products up to 10% concentration available through prescription or over the counter for treatment of acne and rosacea. Acne drug products can also be formulated as a combination of drug products including BPO. Product types include wash-away cleansers and topical creams, gels, and lotions that are left on the skin for long periods of time.
Mass spectrometry methods were used to detect benzene in 111 new, unopened products stored at room temperature on shelves of major US retailers, and the air surrounding them with and without UV exposure. It is the first time BPO drug products have been shown to degrade into benzene via a mechanism other than heat, and furthermore, it shows that benzene formation can occur independently of the starting benzene concentration in new or cold temperature stored BPO drug products.
Implications of Benzene Formation in BPO Treatments
Lead investigator David Light, Co-Founder and President of Valisure, LLC, New Haven, CT, and Affiliate Professor at Arnold & Marie Schwartz College of Pharmacy and Health Sciences, Long Island University, Long Island, NY, notes, “The discovery of benzene formation in benzoyl peroxide acne treatments presents a potentially serious public health risk. Our research demonstrates that these products, widely used by consumers of all ages, can potentially contain or generate concerningly high levels of benzene, particularly under common environmental conditions. This issue highlights the critical importance of rigorous independent testing of drug products to help protect consumers from harmful contaminants or degradation products.”
Expert Opinions on BPO Safety and Regulation
Noted expert Richard L. Gallo, MD, PhD, Department of Dermatology, University of California San Diego, CA adds, “This carefully done analysis should put to rest the question of whether benzene is present in skin care products that contain BPO. It is now important that further studies be conducted to determine if the presence of this potential carcinogen in drugs with BPO translates into any increased risk of cancer.
Commenting on the study, Steve Xu MD MSc, Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, IL, notes, “As the first dermatologist to publish on consumer complaints related to personal care products submitted to the Food and Drug Administration’s Center for Food Safety and Applied Nutrition’s Adverse Event Reporting System, I have long been concerned about the public health safety of these lightly regulated products available in retailers worldwide. BPO products for acne are now the newest addition to a growing list of products that have been recalled or raise safety concerns joining formaldehyde releasing hair care products, benzene contaminated deodorants, and phthalates in shampoos.”
Reference: “Evaluation of Benzene Presence and Formation in Benzoyl Peroxide Drug Products” by Kaury Kucera, Nicola Zenzola, Amber Hudspeth, Mara Dubnicka, Wolfgang Hinz, Christopher G. Bunick, Michael Girardi, Arash Dabestani and David Y. Light, 7 October 2024, Journal of Investigative Dermatology.
DOI: 10.1016/j.jid.2024.09.009