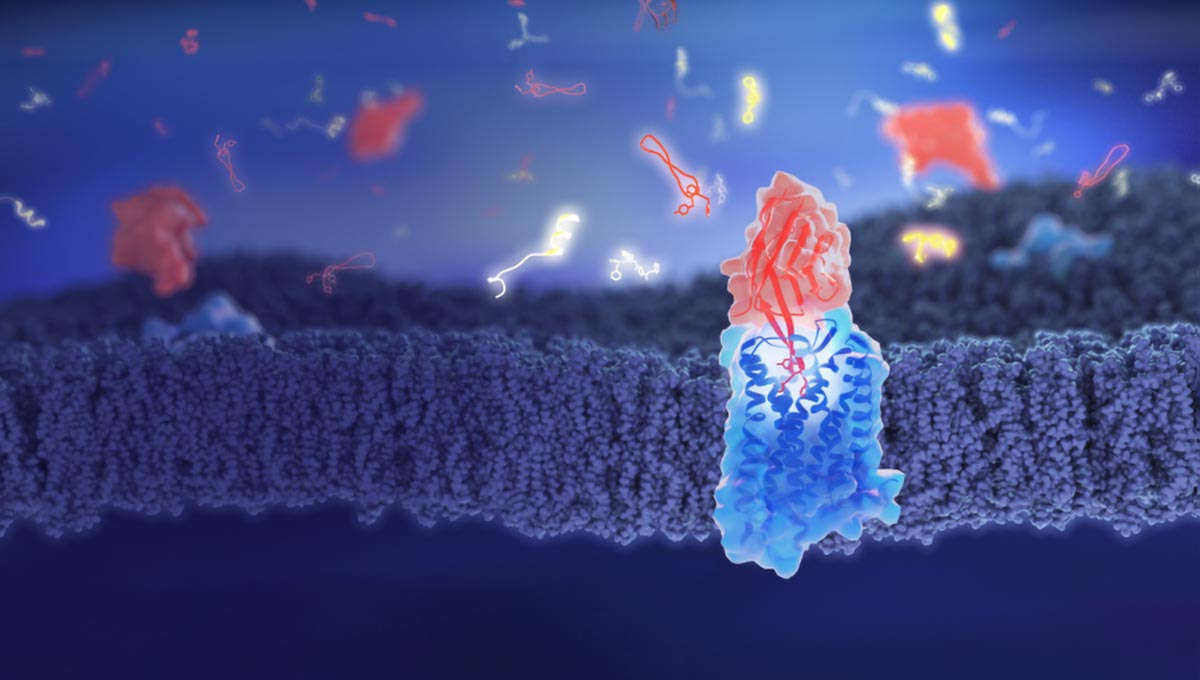
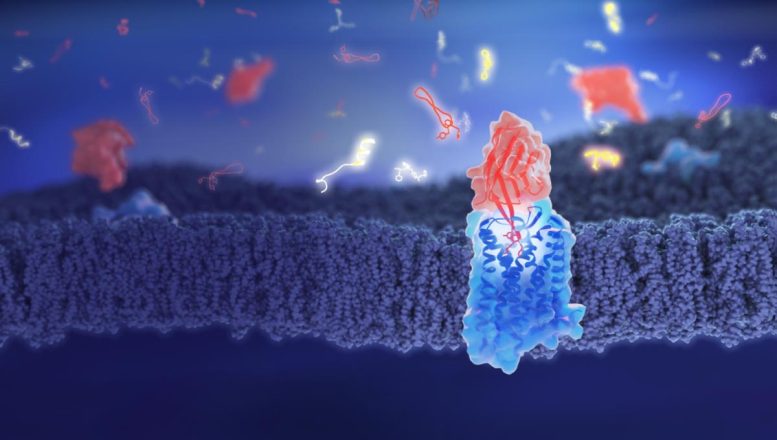
A new molecule, based on llama antibodies, could counteract opioid effects by preventing drugs from binding to brain receptors, thus tackling addiction and overdose issues.
Opioid medications are highly effective pain relievers but are associated with significant risks, including side effects ranging from dizziness to life-threatening respiratory depression. The misuse of these drugs contributes to nearly half a million deaths worldwide annually. Researchers at the University of Geneva (UNIGE) have identified a molecule, named nanobody NbE, that binds strongly and persistently to the same cell receptors as opioids, effectively blocking their activity. The team has also developed even smaller molecules that maintain these properties, potentially offering a far more effective means than current methods for reducing the adverse effects of opioids. These findings were reported in the journal Nature Communications.
The Opioid Crisis
Opioids, which encompass drugs like morphine, fentanyl, and tramadol, are potent painkillers that also induce euphoria by interacting with brain nerve cells. Their highly addictive nature and severe side effects pose significant health risks. Misuse of both natural and synthetic opioids has led to them becoming the most lethal drugs in the United States, with the crisis now expanding to Europe.
“Our new molecules have the potential to reverse or reduce the deleterious side effects of opioids.”
“We need to urgently develop new molecules to better mitigate the side effects for patients and manage the risks of opioid-related overdoses,” explains Miriam Stoeber, associate professor in the Department of Cell Physiology and Metabolism at UNIGE Faculty of Medicine, who initiated and coordinated the project. “To understand how a molecule works, we need to know how it affects the brain cells. In our study, we used tiny natural proteins derived from llama antibodies, called nanobodies, designed to bind specifically to the target receptor on the cell’s surface.”
Enhanced Binding and Blocking by the Nanobody NbE
UNIGE researchers have found that NbE, one of the nanobodies under study, has the unique ability to bind so tightly and durably to specific opioid receptors that it prevents opioids from binding to these same receptors, therefore blocking the drug’s activity. “To determine how NbE binds to its target, we used high-resolution structural biology methods, thanks to the new Dubochet Centre for Imaging,” describes Andreas Boland, assistant professor in the Department of Molecular and Cellular Biology at UNIGE Faculty of Science, and co-last author of the study.
“We identified a unique binding mode where only a small portion of the nanobody is responsible for its correct receptor selectivity. Knowing precisely which part of the nanobody is at stake allows us to imagine new ways to induce the same effects with pharmaceuticals.”
Development of Smaller, More Effective Molecules
While significantly smaller than antibodies, nanobodies remain quite large. They can be costly to produce and may not fully reach the target tissue in the body. In collaboration with Prof. Steven Ballet’s team from the University of Brussels, the UNIGE research team synthesized in vitro a set of even smaller molecules mimicking the key part of NbE responsible for the selected binding to opioid receptors.
“By durably blocking opioid receptors, our new molecules have the potential to reverse or reduce the deleterious side effects of opioids. In case of overdose, they could provide a better, longer-lasting option than naloxone, the treatment currently in use. We will now refine their structure to improve even further their efficiency and facilitate their delivery to the targeted nerve cells in the brain,” concludes Miriam Stoeber.
Reference: “Structural basis of μ-opioid receptor targeting by a nanobody antagonist” by Jun Yu, Amit Kumar, Xuefeng Zhang, Charlotte Martin, Kevin Van holsbeeck, Pierre Raia, Antoine Koehl, Toon Laeremans, Jan Steyaert, Aashish Manglik, Steven Ballet, Andreas Boland and Miriam Stoeber, 9 October 2024, Nature Communications.
DOI: 10.1038/s41467-024-52947-6