

Cryo-EM and MD simulations reveal the mechanism by which the potent neurotoxin α-latrotoxin forms calcium-permeable membrane pores.
The black widow spider is one of the most feared spider species. Its venom is a cocktail of seven different toxins that attack the nervous system. These so-called latrotoxins specifically paralyze insects and crustaceans. However, one of these toxins, α-latrotoxin, specifically targets vertebrates and is toxic to humans. It disrupts the transmission of nerve signals, leading to its harmful effects.
As soon as α-latrotoxin binds to specific receptors of the synapses – the contacts between nerve cells or between nerve cells and muscles – calcium ions flow uncontrollably into the presynaptic membranes of the signaling cells. This induces the release of neurotransmitters, triggering strong muscle contractions and spasms.
Complexity of the Process
Despite the apparent simplicity of this process, there is a highly complex mechanism behind it. Scientists at the University of Münster (Germany) have now deciphered the structure of α-latrotoxin before and after membrane insertion at near-atomic resolution.
In order to better understand the mechanism of calcium influx into the presynaptic membrane, experts from the Center for Soft Nanoscience at the University of Münster, headed by Prof Christos Gatsogiannis (Institute of Medical Physics and Biophysics) and Prof Andreas Heuer (Institute of Physical Chemistry), used high-performance cryo-electron microscopy (cryo-EM) and molecular dynamics (MD) computer simulations.
Transformation of the Toxin
They showed that the toxin undergoes a remarkable transformation when it binds to the receptor. Part of the toxic molecule forms a stalk that penetrates the cell membrane like a syringe. As a special feature, this stalk forms a small pore in the membrane that functions as a calcium channel. MD simulations revealed that calcium ions can flow into the cell through a selective gate located on the side directly above the pore.
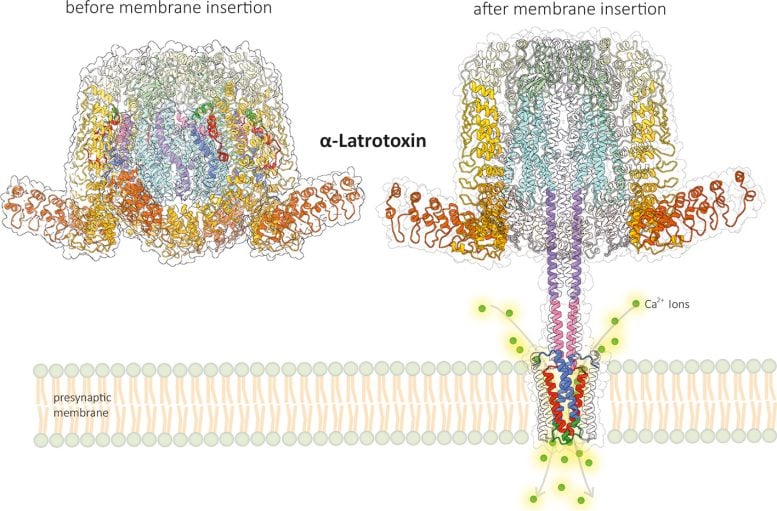
Thanks to these results, researchers now better understand how α-latrotoxin works. “The toxin mimics the function of the calcium channels of the presynaptic membrane in a highly complex way,” explains Christos Gatsogiannis. “It therefore differs in every respect from all previously known toxins.” The new findings open up a wide range of potential applications; latrotoxins have considerable biotechnological potential, including the development of improved antidotes, treatments for paralysis, and new biopesticides.
The research results have just been published in the journal Nature Communications. In previous work, the research group led by Christos Gatsogiannis had already deciphered the structure of insect-specific latrotoxins in the venom of the black widow spider before inserting into the membrane.
Reference: “Structural basis of α-latrotoxin transition to a cation-selective pore” by B. U. Klink, A. Alavizargar, K. S. Kalyankumar, M. Chen, A. Heuer and C. Gatsogiannis, 3 October 2024, Nature Communications.
DOI: 10.1038/s41467-024-52635-5
The study was funded by the German Research Foundation.