Antimicrobial resistance (AMR) bacterial infections have become a serious problem threatening human health worldwide. The overuse of antibiotics has promoted drug-resistant mutations in bacteria, causing almost all clinically used antibiotics to develop resistance in different strains.
In May 2024, the World Health Organization (WHO) updated its list of drug-resistant bacteria that pose the greatest threat to human health, among which Pseudomonas aeruginosa was listed as a high-priority pathogen. Pseudomonas aeruginosa is a particularly dangerous bacterium that can cause a wide range of infections, including pneumonia, urinary tract infections, and bloodstream infections.
It is known for its ability to develop resistance to antibiotics quickly, making it challenging to treat and posing a significant risk to immunocompromised patients, such as those in hospitals or with chronic illnesses. Given the urgency of the situation, it is essential to develop new treatment strategies to combat superbug infections.
Recently, a team led by Professor Hongzhe Sun from the Department of Chemistry at The University of Hong Kong, in collaboration with the University of Groningen (Netherlands) and Nankai University, has made a breakthrough in solving this challenging problem. Their work has been published in Nature Microbiology.
The team’s research has shown the combination of different types of antibiotics with bismuth-based drugs (such as bismuth subsalicylate, commonly known as Pepto-Bismol) disrupts bacterial iron homeostasis, effectively restoring the bactericidal function of multiple antibiotics. This combination therapy leads to the elimination of multi-drug resistant bacteria Pseudomonas aeruginosa.
Their efficacy has been demonstrated in both bacterial-infected cells and in mouse models, providing crucial strategies for combating the global threat of antibiotic resistance (AMR) and offering potential clinical applications.
Professor Hongzhe Sun stated, “The next epidemic is likely to be an infection caused by superbugs. We must be fully prepared for it and have powerful arsenals to treat such deadly infections.”
Metal compounds have traditionally been used as antibacterial agents. Their multi-target mode of action allows them to interfere with multiple biological pathways in pathogens, resulting in a lower frequency of drug resistance. In recent years, Professor Sun’s team has carried out extensive work to address the problem of “superbugs.” For example, metallodrugs such as bismuth citrate have effectively inhibited the activity of metallo-β-lactamase, an important enzyme that bacteria use to destroy commonly used antibiotics.
His team also employed a dual “Trojan Horse” strategy, using metallo-sideromycin to restore the activity of Cefiderocol, one of the latest antibiotics. Cefiderocol is “smuggled” into bacterial cells through the bacteria’s uptake of ferric iron (Fe3+), enhancing its bactericidal effect. Additionally, a bismuth drug mimicking ferric iron enters bacteria cells and inhibits key bacterial enzymes, achieving a dual function.
Developing new antibiotics is both costly and time-consuming, while bacteria are quickly developing resistance, shortening the lifespan of effective antibiotics. As a result, combining existing drugs synergistically is emerging as a powerful alternative to combat the rise and spread of antibiotic-resistant bacterial strains.
This study demonstrates that bismuth disrupts iron homeostasis by specifically binding to siderophore (molecules used by bacteria to obtain iron) and iron absorption regulator Fur in Pseudomonas aeruginosa.
Within bacterial cells, bismuth specifically targets iron-sulfur cluster enzymes, leading to the inhibition of respiratory complexes. This disruption impairs the electron transport chain and dissipates the proton motive force. Consequently, the activity of export pumps is damaged, resulting in the accumulation of antibiotics within the bacteria and enhancing their efficacy.
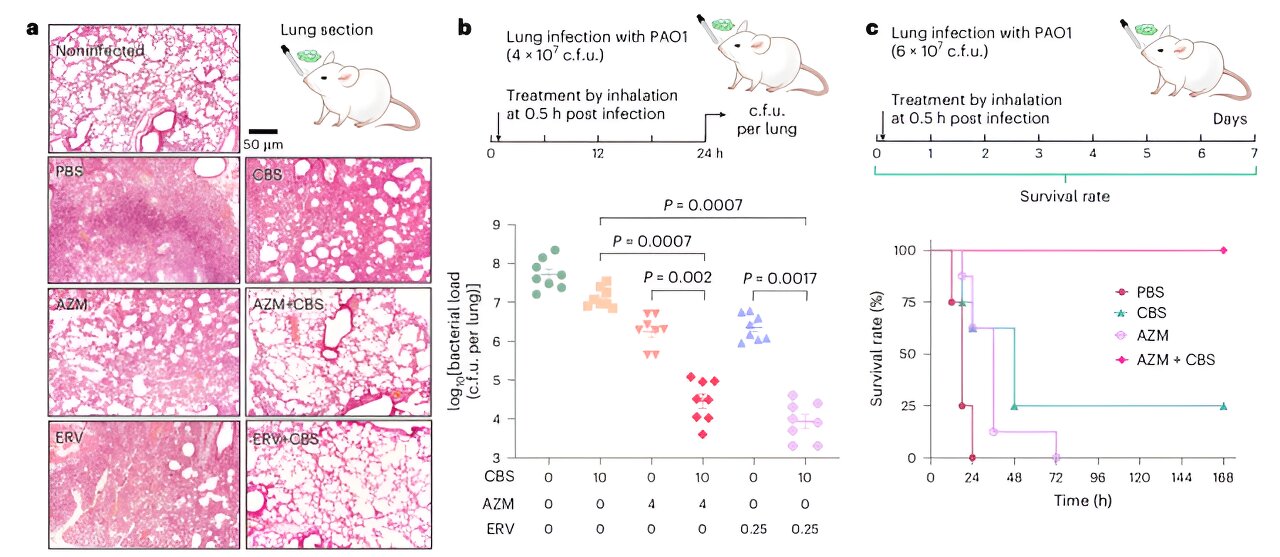
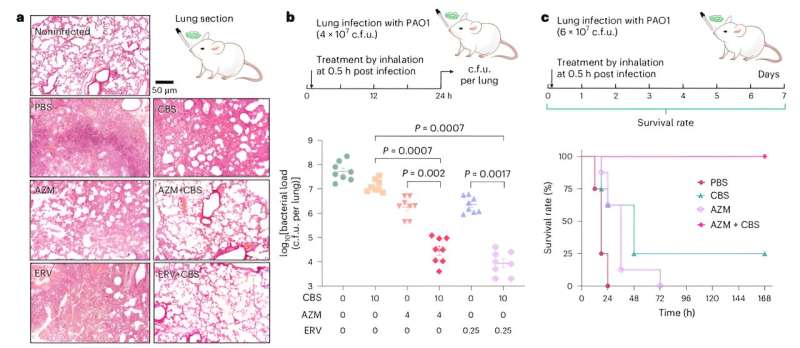
At the same time, this approach also enhances the killing effect of antibiotics on bacteria within the biofilm. The combined treatment also demonstrated powerful antibacterial effects in the bacterial infected cell models, and the team verified the effectiveness of this therapy in more than 100 strains of clinically resistant Pseudomonas aeruginosa.
Finally, in a mouse lung infection model, the treatment significantly reduced bacterial colonization in the lungs and improved the survival rate of mice. These findings lay a solid foundation for future efforts to combat drug-resistant bacterial infections and pave the way for further clinical applications.
More information:
Yushan Xia et al, Bismuth-based drugs sensitize Pseudomonas aeruginosa to multiple antibiotics by disrupting iron homeostasis, Nature Microbiology (2024). DOI: 10.1038/s41564-024-01807-6
Provided by
The University of Hong Kong
Citation:
Research team develops metallodrug-antibiotic combination strategy to combat superbugs (2024, October 8)
retrieved 9 October 2024
from https://phys.org/news/2024-10-team-metallodrug-antibiotic-combination-strategy.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.