Christian de Caestecker, a Ph.D. student in the lab of Ian Macara, Louise B. McGavock Professor and chair of the Department of Cell and Developmental Biology, has proposed and validated a mechanism that addresses a decades-old mystery surrounding epithelial cells. de Caestecker’s research, published in Nature Cell Biology, sheds light on the process by which epithelial cells, polarized cells that face the outside world, sort and deliver the specialized proteins they need at each cell’s top (outermost) surface.
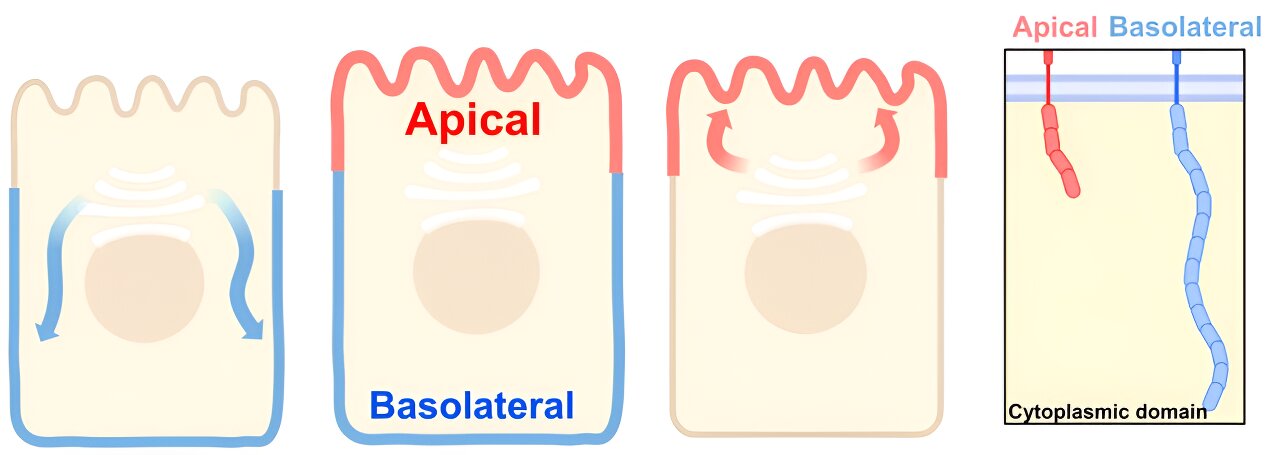
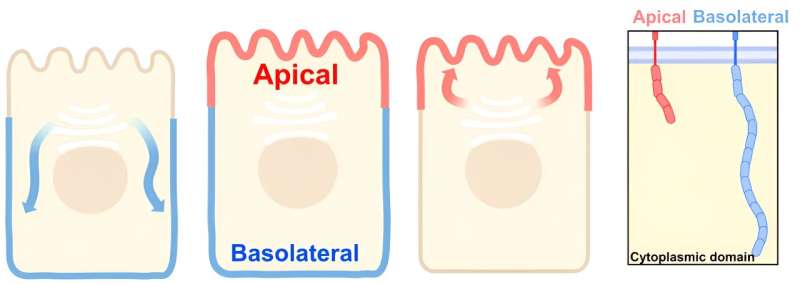
Epithelial cells are organized like boxes, with tops and sides called the apical and lateral surfaces. To properly perform their functions, epithelial cells must accurately sort proteins to each surface, and defects in the delivery of such proteins can play a role in multiple human diseases such as cancers.
“Most human cancers are epithelial in origin and show defects in polarized membrane protein distributions,” Macara said. “How delivery occurs to the top, or apical, surface has remained a mystery for decades, but Christian’s brilliance and hard work led to an important breakthrough.”
Freshly synthesized proteins are sent to the Golgi for additional processing and for sorting to their final destinations through a process analogous to the postal service. Delivery to the sides of the cell is well understood, owing to the presence of “zip codes” in proteins that instruct the cell which path the protein should take to the cell surface.
Apical proteins lack equivalent zip codes, so the apical sorting process has remained more elusive. Considering that many apical membrane proteins have very short or non-existent cytoplasmic domains—meaning that most of their bulk resides within or outside of the plasma membrane and not inside the cell—de Caestecker hypothesized that proteins destined to go to the apical membrane are sorted at the Golgi by the physical size of the cytoplasmic domains.
To test this idea, he looked at three representative apical proteins with short cytoplasmic tails and used synthetic biology approaches to change the size of their tails and observe how this affected sorting. de Caestecker found that, when their cytoplasmic tails were lengthened, the proteins experienced a significant delay in processing and exit from the Golgi and were misdelivered to the sides of the cell instead of the top.
Moreover, using super-resolution microscopy at the Cell Imaging Shared Resource, they observed segregation of the small and bulky cargoes to distinct regions of the Golgi during trafficking, suggesting that a large cytoplasmic tail blocks access to specialized domains within the Golgi that may be involved in deliveries to the apical membrane.
de Caestecker also tested whether apical proteins that have binding partners are sorted individually or bound and found that, although the proteins and their binding partners co-traffic to the Golgi, they dissociate before getting trafficked to the apical membrane. These results support the hypothesis that the Golgi uses a size filter to determine if a protein should be trafficked to the apical membrane or not.
“How cells sort and deliver polarized proteins to their appropriate domains is a fundamental question in cell biology,” de Caestecker said. “We need to first understand this process in normal cells so that we can identify how it is disrupted in diseases such as cancers.”
More information:
Christian de Caestecker et al, A size filter at the Golgi regulates apical membrane protein sorting, Nature Cell Biology (2024). DOI: 10.1038/s41556-024-01500-0
Provided by
Vanderbilt University School of Medicine Basic Sciences
Citation:
Shedding light on a decades-old protein sorting mystery (2024, September 26)
retrieved 26 September 2024
from https://phys.org/news/2024-09-decades-protein-mystery.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.