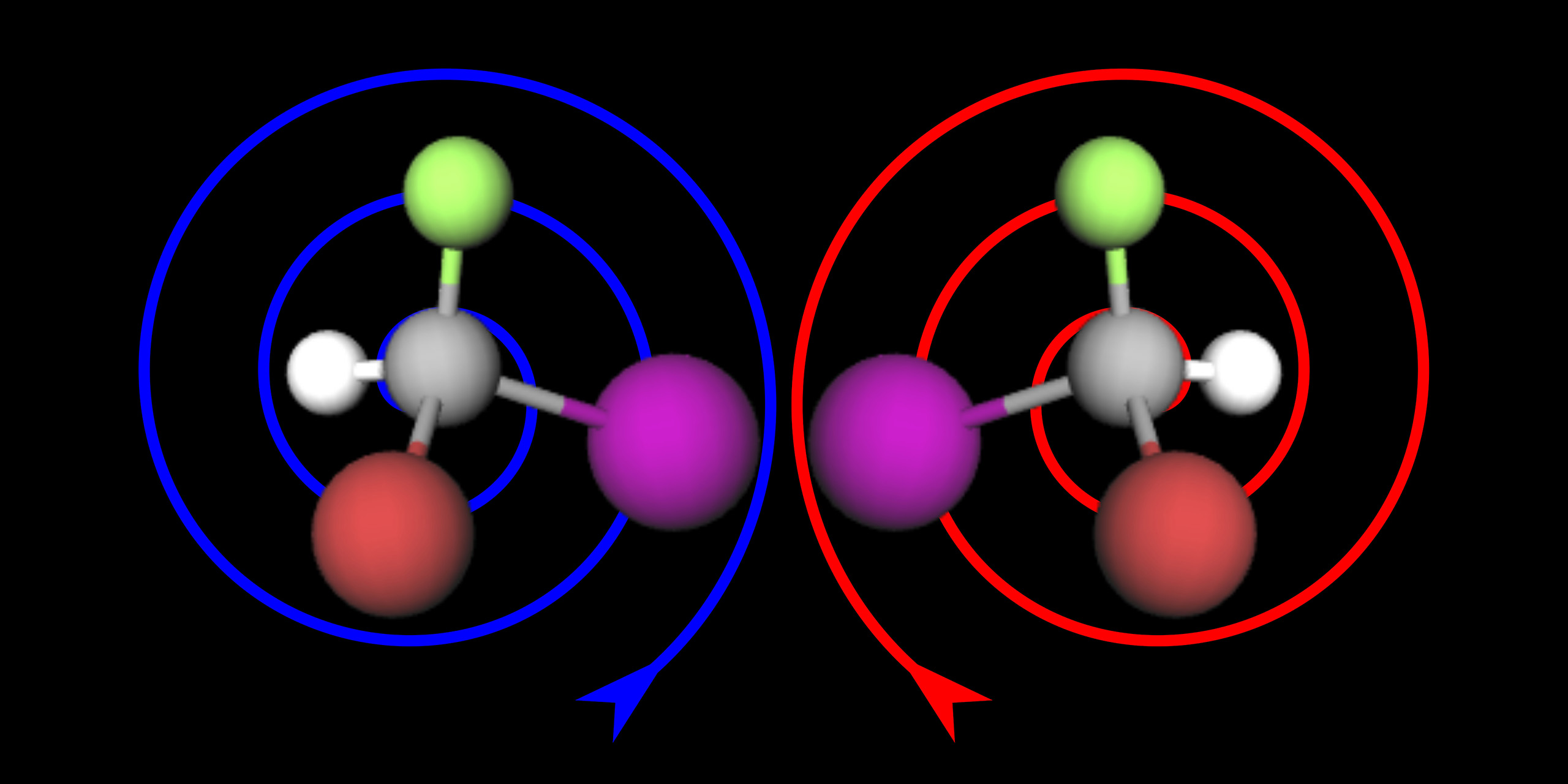
Researchers from KIT and Voxalytic GmbH have developed a new method that simplifies the process of mapping the spatial arrangement of atoms, offering a valuable tool for drug discovery.
Chirality in a molecule refers to its fundamental structure, where certain molecules, known as enantiomers, exist in pairs as mirror images of one another. They are comparable to the way a left glove differs from a right glove. The “twist” of the molecule, whether left-handed or right-handed, affects how it interacts with biochemical and chemical reactions. Despite their mirror-image relationship, the properties of these enantiomers can vary significantly, sometimes even producing opposing effects.
With drugs, this can have devastating consequences: When children were born with physical deformities in Germany and England in 1960, the active ingredient called “Contergan” or “Thalidomid” was the cause. The drug had been administered to pregnant women for the treatment of pregnancy disorders and was subsequently banned. Since then,it is compulsory for pharmaceutical companies to check whether the active ingredients, which are often chiral, are not converted to their opposite enantiomer in the human body.
Method Facilitates Search for Active Ingredients
Now a joint team of KIT and Voxalytic GmbH (a spin-off from KIT and the University of Freiburg), led by Professor Jan Korvink, Director of the Institute of Microstructure Technology at KIT, has succeeded in measuring the chiral molecular structure directly using nuclear magnetic resonance (NMR) spectroscopy.
Although NMR spectroscopy is the only method to elucidate chemical structures down to atomic resolution at ambient temperature, it has so far been “blind” to the chirality of molecules. In order to measure the “twists” of a molecule, an optical method has been used up to now that can recognize the sense of rotation, but not at atomic resolution.
“We are very excited about the opportunity to turn this method into a convenient tool for the industry and have had the concept patented,” says Jan Korvink. This could make chirality elucidation a future standard method of NMR analysis, as Dr. Sagar Wadhwa from Voxalytic, who wrote his doctoral thesis on this topic, explains. “This will make work easier for chemists who do research into the production of specific enantiomers.” Dr. Dominque Buyens, biochemist and postdoctoral researcher at KIT, adds: “We will study the new method in the context of drug development. It could significantly accelerate drug screening.”
Reference: “Direct Chiral Discrimination with NMR” by Sagar Wadhwa, Dominique Buyens and Jan G. Korvink, 23 August 2024, Advanced Materials.
DOI: 10.1002/adma.202408547
The KIT team is funded by an ERC Synergy Grant (HiSCORE), the CRC HyPERiON, and the VIRTMAT Helmholtz Association.