
When a rash broke out on the hand of a patient taking part in the trial of a promising schizophrenia drug, clinical investigator Greg Mattingly began to fret that it might be an allergic reaction.
Instead, it turned out to be a mark of success: the patient, in his fifties, had landed his first job in more than a decade as a waiter, and the skin inflammation was a side effect not of the drug, but of washing dishes.
Securing a full-time job may seem unremarkable, but for most people living with schizophrenia, it can represent a triumph against the odds.
Schizophrenia sufferers are frequently pushed to the fringes of society, haunted by the delusions and hallucinations that define the worst flare-ups of the illness, while poorly served by a choice of old and imperfect treatments. In the US, they are vastly over-represented in the jobless, homeless and prison populations.
Now hope is at hand. If approved by regulators at the US Food and Drug Administration later this month, the experimental drug, known as KarXT, a twice daily pill, will arguably be the first truly novel treatment for schizophrenia in more than seven decades.
Existing medicines serve to do little more than sedate patients or are undermined by a long list of side effects. “A shot at a normal life has been out of reach for most patients,” says Mattingly, who has overseen more than 400 trials testing psychiatry drugs.
Stemming from the ancient Greek for split mind, schizophrenia typically emerges in late adolescence as a result of genetic predisposition, combined with environmental factors such as stress or drug use. Sufferers can experience symptoms ranging from scrambled short-term memories and social isolation, to psychotic breakdowns lasting for weeks.
Neuroscientist Steve Paul, who developed the precursor to KarXT while working at Eli Lilly, describes schizophrenia as the “cancer of psychiatry”, because of the damage it does to sufferers, which can result in their lives being cut short by almost 30 years compared with the average life expectancy.
Globally, about 24mn people, or one in every 300, are affected by the disease. However, in the US rates are thought to be even higher — with estimates of prevalence ranging from 0.25 per cent to as high as 1.6 per cent, or 3.8mn adults.
So severe are problems faced by those with psychiatric illnesses, including anxiety, depression and in the most extreme cases schizophrenia, the US government has said the country is in the grip of a mental health crisis. Schizophrenia alone costs the US $170bn a year.

New solutions to America’s mental health crisis could be on the horizon, however. KarXT is widely expected to receive regulatory approval in the US, which could pave the way for its rollout in global markets. Big Pharma has renewed enthusiasm for neuroscience, illustrated by Bristol Myers Squibb’s $14bn acquisition of Karuna Therapeutics, the biotech behind KarXT, announced at the end of last year.
But innovative medicines butt up in the US against a healthcare system ill-equipped to provide adequate psychiatric care, especially for the most serious illnesses such as schizophrenia.
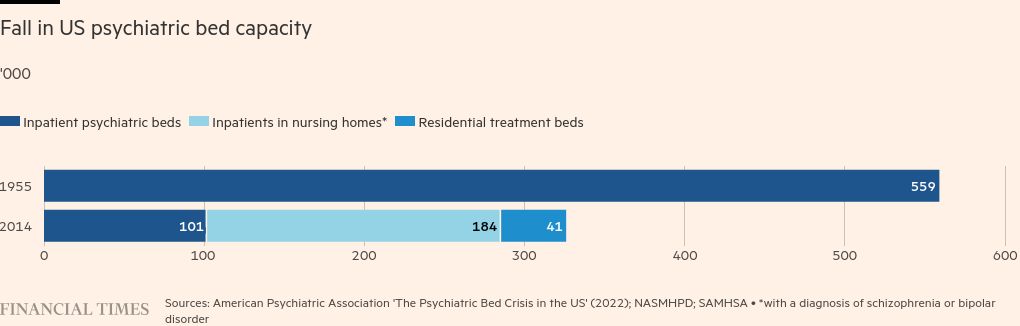
A relentless decline in psychiatric bed capacity has left patients adrift, leaving prisons and jails as the de facto mental health institutions, and there is also a growing shortage of psychiatrists.
The cost and complexity of the US healthcare system are also a challenge. The Institute for Clinical and Economic Review, an influential drug pricing non-profit, recommended that KarXT is priced up to $20,000 a year, but such a high price may limit access.
“How much can new therapeutics solve this crisis when you have an underfunded mental health system with an inadequate workforce and a chaotic melange of services? It’s a piece of the puzzle but it’s not a panacea,” says Ken Duckworth, chief medical officer of the National Alliance on Mental Illness, a non-profit.
With 86bn neurons, the brain is the most complex human organ — and has proved to be science’s most elusive target for treatment.
Animal models, which seek to first test medicines in mice, have faltered with psychiatrist drugs because of the uniquely intricate circuitry of the human brain. “The thing that really makes us human is what is the root cause of schizophrenia,” says Alan Breier, a psychiatry professor at Indiana University and former chief medical officer at Eli Lilly. “How do you get an animal model for human thought and human emotion? It’s very difficult.”
Consequently, the biggest breakthroughs in schizophrenia drug development have a habit of happening by accident.
While testing out allergy medication chlorpromazine as a painkiller in the 1950s, French military surgeon Henri Laborit realised that the drug also dulled the frightening delusions of soldiers in the grip of psychosis, by blocking receptors of the feel-good chemical dopamine, excess levels of which are linked to schizophrenia.
The discovery offered the first alternative to the crude lobotomy procedure as well as to the reflex to lock schizophrenia patients up in asylums.
Similarly, after Eli Lilly scientists developed the company’s first experimental Alzheimer’s drug to enter human trials — xanomeline — in the 1990s, they were surprised to discover during clinical trials that it reduced the psychosis symptoms experienced by about a third of patients with the memory-robbing disease.
More than a decade later, this drug would be licensed by Karuna’s founders and combined with another drug, trospium, to quell gastrointestinal side effects, creating KarXT. The drug works by targeting muscarinic receptors in the brain that modulate dopamine release at the source, rather than blocking dopamine directly.
But the current generation of schizophrenia medications, often hailed as big advancements by drugmakers when they were released and mostly known as atypicals, are really just iterations of chlorpromazine.
They often succeed in stopping psychotic episodes, but leave patients listless and withdrawn, and prone to rapid weight gain. Up to half of patients on traditional antipsychotics end up having a disorder known as tardive dyskinesia, which causes involuntary, uncontrollable movements.
Like most men with schizophrenia, Robert from Cleveland, Ohio, first developed symptoms in his early twenties.
Robert, now in his late twenties, dropped out of university a semester before graduation as the illness began to take hold. He has now tried four different existing drugs to tame his symptoms, including most recently Invega and Lamictal.
But his mother, Jill, a trained psychotherapist, says he dislikes the medication and is reluctant to take it. The problem for many patients, explains Henry Nasrallah, an emeritus professor of psychiatry at the University of Cincinnati, is not a lack of access to medication, “it’s simply that they deny they’re even sick” — a condition known as anosognosia.
For Jill, helping her son acknowledge the depth of his illness is a slow process. “We don’t have to agree on the treatment options, but we can agree that you want to get to this point where you can live alone and have a meaningful life,” says Jill. “Right now, he’s thinking, ‘I’m good enough, I hate medications, I don’t want to try anything new.’”
Robert spends most of his time locked away in his childhood bedroom, broken up by the occasional outing to a restaurant or a bowling alley with his parents. Robert and Jill have both been identified by a pseudonym, due to the continued stigma around the disease.
Jill says she is open to her son Robert trying KarXT as it has been shown to better combat symptoms of anhedonia, which leaves patients unable to experience pleasure, and resolve cognitive impairment including poor memory retention.
But she remains cautious, in large part because of her mistrust of Big Pharma. “We’re talking about my son’s wellbeing, this isn’t about treating cholesterol,” says Jill. “If this drug is as they say, then great but there’s a vested monetary interest for the pharmaceutical industry to get this drug out there. They have billions of dollars invested in this; I have my son’s livelihood at stake.”
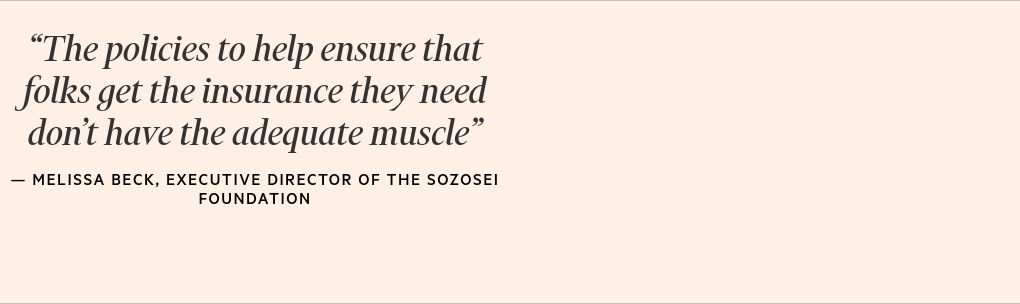
The risks are high for both patients and drugmakers. Many companies, including Pfizer and Bristol Myers Squibb, had backed away from developing neuroscience drugs, in large part because of the high failure rate in clinical trials resulting from the struggles that animal models face in accounting for the brain’s complexity.
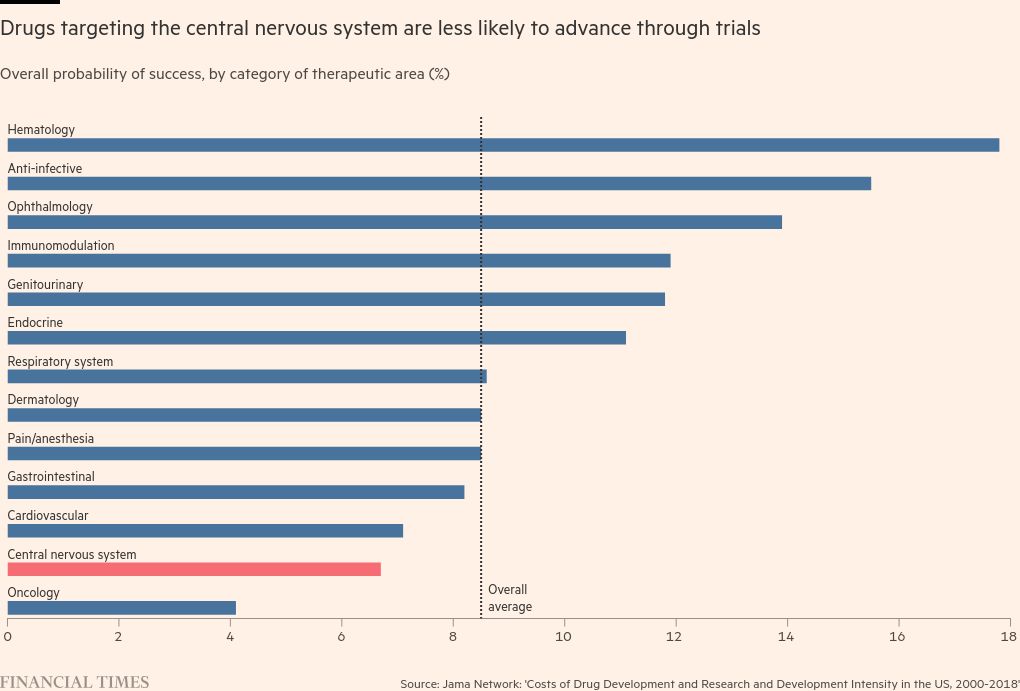
Of the 13 main therapeutic areas, drugs targeting the central nervous system have the second lowest probability of making it from pre-clinical trials to approval, succeeding just 6.7 per cent of the time, according to a study published by Harvard researchers in the Jama medical journal this year.
Such disorders “are among the most common afflictions of humankind but drug development has been slow and disappointing over the past few decades”, says Paul, who served as CEO of Karuna from 2018 until its acquisition by BMS last year. “But things are starting to change.”
In addition to BMS’s acquisition of Karuna at the end of last year, AbbVie spent $8.7bn on neuroscience biotech Cerevel Therapeutics, which is developing a similar schizophrenia drug, known as emracladine, as well as treatments for epilepsy and Parkinson’s — marking the two biggest psychiatric drug investments in at least a decade.
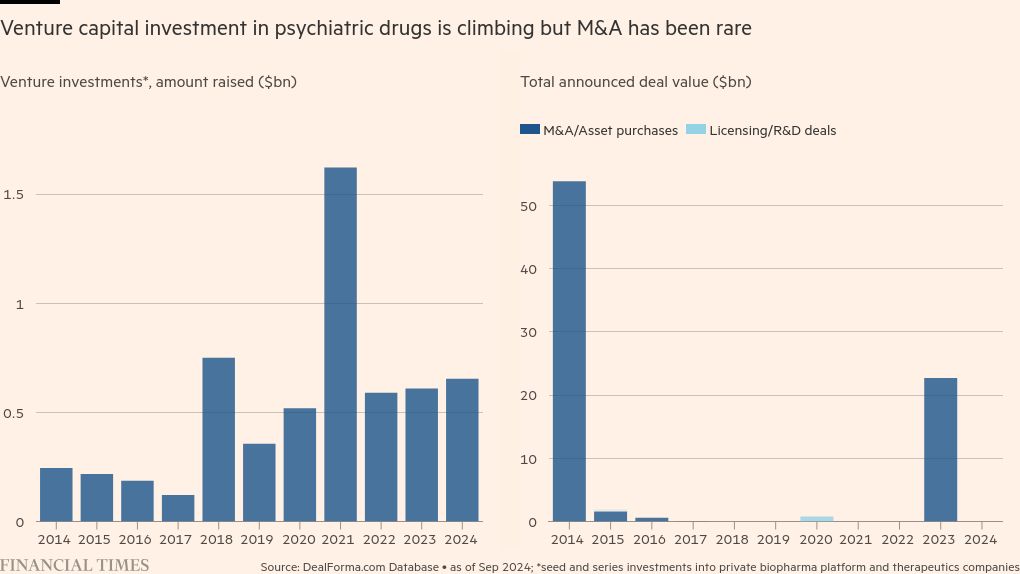
Venture capital investment in psychiatric drugs is also on course to record its second-highest year ever, with $654mn deployed so far, according to biopharma data platform DealForma. Moreover, the first two fully approved Alzheimer’s drugs in the US were launched in the past year.
Roopal Thakkar, AbbVie’s chief scientific officer, says drugmakers are “getting better at cracking that code”, adding that improvements in brain imaging and the ability to combine different molecules to improve efficacy and reduce side effects mean there is an “opportunity here to go deeper than before”.
BMS’s chief medical officer, Samit Hirawat, agrees: “There is renewed interest because we understand the biology even better so I do believe in the coming years we should be able to see more successes.”
Paul Matteis, an analyst at investment bank Stifel, says this uptick in investment is only likely to continue as “the mental health epidemic creates diseases of mass markets”. “You’re talking about tens of millions of people with anxiety and depression, and millions of people with schizophrenia,” he says.
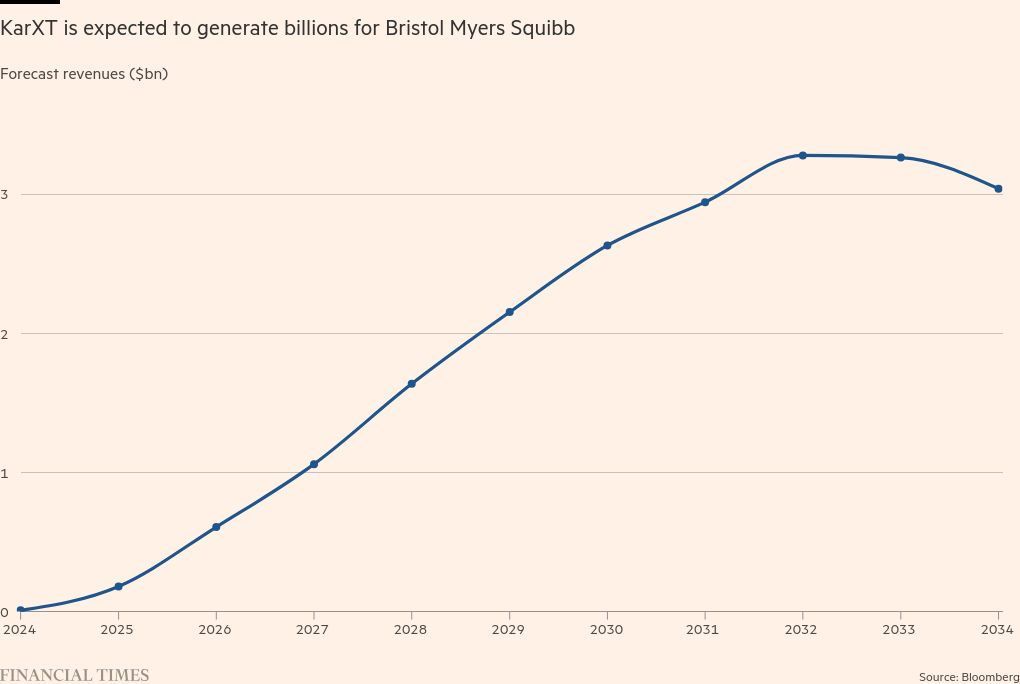
KarXT is projected to generate $8.2bn in revenues for BMS by the end of the decade, according to analysts’ consensus estimates.
But access to new schizophrenia drugs for those who most need them is likely to remain spotty, as they compete with cheap generic versions of atypicals, which are often prioritised by insurers’ drug formularies.
This is particularly the case in US correctional facilities, where budgets are stretched but up to 4 per cent of the population have schizophrenia, far higher than the rate in the population at large, studies suggest.
“While science may be growing by leaps and bounds, the policies, laws and regulations in place to help ensure that folks get the insurance they need to cover those treatments don’t have the adequate muscle,” says Melissa Beck, executive director of the Sozosei Foundation, the charitable arm of drugmaker Otsuka, which is focused on what it calls “decriminalising mental health” in the US.

The fact that some people are diagnosed with mental illness only when they are imprisoned speaks to a broader problem in the US with the treatment of the mentally ill by police and the justice system.
Earlier this summer, when Robert stopped taking the drugs he was prescribed, he spiralled into a manic episode, landing himself in the emergency room.
He found himself flanked by six police officers surrounding his gurney, who eventually resorted to tasering him to get him under control. “It was just incredibly poor care,” says his mother, Jill.
Mentally ill patients being met with the heavy hand of the law is an all too familiar tale for Steve Leifman, an associate administrative judge in Florida’s Miami-Dade County. “I see more people in a day with serious mental illnesses in my criminal division than most psychiatrists will see in a month,” he estimates.
A study carried out in Miami-Dade County found that between 1985 and 2023, the same 97 individuals with severe mental illnesses, including schizophrenia, had been arrested 4,210 times and spent nearly 100,000 days in prison between them, costing the taxpayer $12.6mn in jail stays and hospital visits. “We are in a situation where we are endangering public safety, we are wasting critical tax dollars and we are hurting people,” says Leifman.
Despite the costs to society of leaving severe mental health problems untreated, the access to psychiatric care for those most in need is lacking, particularly for homeless people, about 10 per cent of whom suffer from schizophrenia, according to official estimates.
“That’s a frustration that a lot of people have,” says Michael Thompson, a criminal justice expert who previously worked at the non-profit Council of State Governments. If you are in jail you have a constitutional right to healthcare, “whereas if you are on the streets, you don’t”.
Even for patients with health insurance, getting access to a psychiatrist often proves more difficult than accessing other types of physician.
Reimbursement rates, a key lever that insurance plans use to encourage healthcare providers to join their network, were 22 per cent higher among medical and surgical physicians than psychiatrists in 2021, according to a report published this year by the non-profit RTI International.
As of last year, inpatient capacity in state psychiatric hospitals had fallen to a historic low, according to the Treatment Advocacy Center.
The problems with how psychiatric care is provided in the US have not entirely dulled enthusiasm for KarXT’s launch, however.
Hirawat of BMS estimates that 85 per cent of schizophrenia patients will be covered by Medicare and Medicaid, the state-backed healthcare programmes for the elderly and underprivileged, which he expects to cover KarXT.
“Because many of these patients are already on antipsychotics, there’s a huge bolus of patients who’ve already tested out the generics that are available out there,” says Hirawat.
The arrival of a medication tailored to treat schizophrenia rather than a so-called “antipsychotic” will help to reduce stigma, says the clinical investigator Greg Mattingly. “The term antipsychotic has a lot of stigma with patients and with families, so getting rid of that alone will be a breakthrough.”
After a year on KarXT, Mattingly’s patient had to stop taking the treatment as the open-label trial, a study where participants are told they are on the drug, came to an end. However, he has been phoning Mattingly almost every week asking when the drug will finally be approved.
“We used to talk about keeping people safe, now we’re talking about job training, cooking meals and exercise — that’s not stuff we talked about 20 years ago in schizophrenia,” says Mattingly.