Researchers have developed a tool that provides new insights into cause-and-effect relationships between cells and how these change over time.
The research, published today as a Reviewed Preprint in eLife, is described by the editors as a fundamental study presenting a new data-processing pipeline that could be used to better understand cell–cell interactions. The utility of this pipeline is convincingly illustrated using tumor-on-chip ecosystem data, but it could also be applied to perform causal discovery in other areas of science, meaning this work could potentially have a wide range of applications.
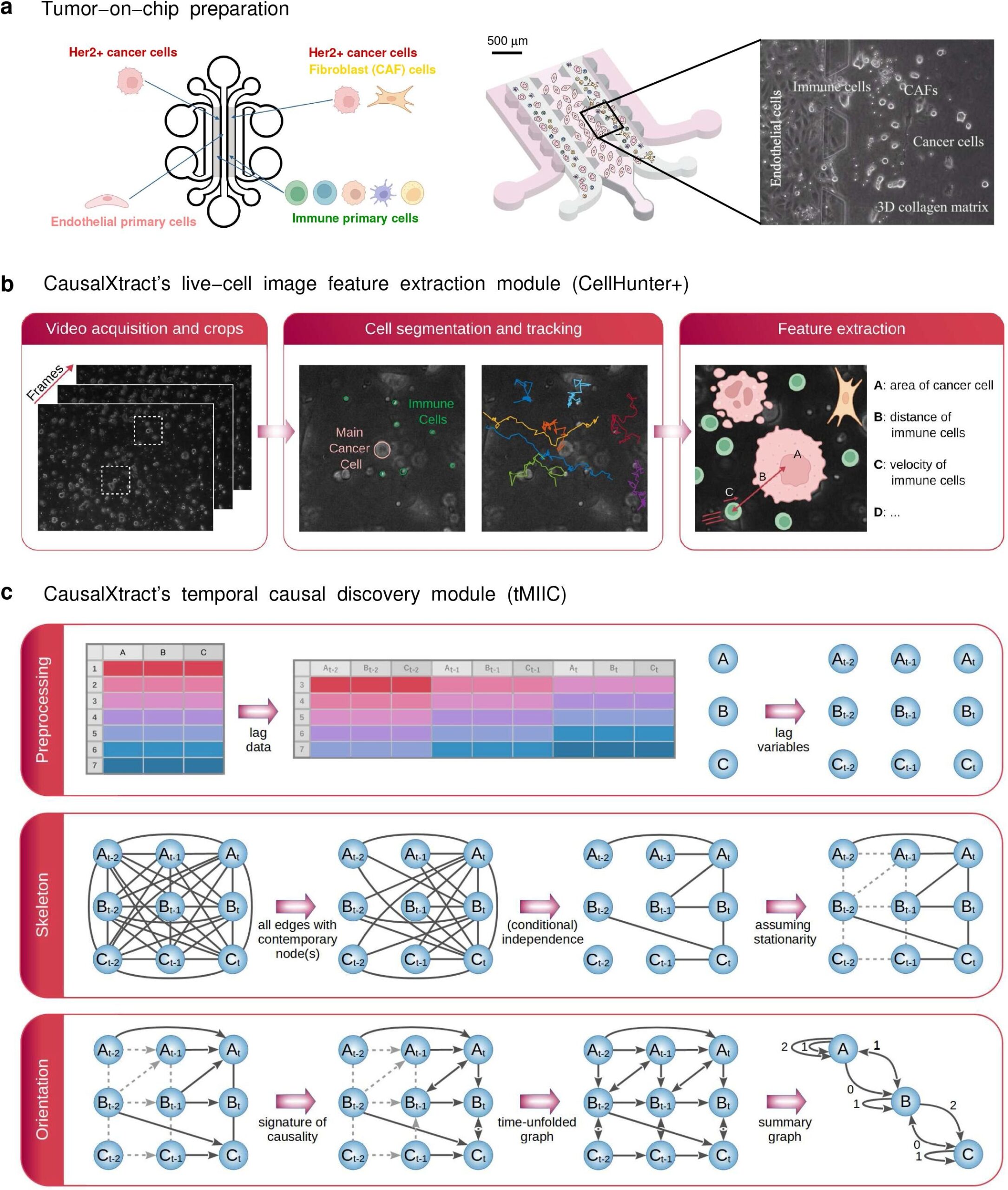
The ability to obtain images of live cells under different experimental conditions has made it possible to extract invaluable information about the shape and status of cells and their interactions with other cells. But this wealth of information remains underexploited because, until now, there has been a lack of methods and tools that can pinpoint the cause-and-effect relationships between the features seen. This ability to pinpoint cause-and-effect is called causal discovery.
The new tool, called CausalXtract, was adapted from a previous discovery method which can learn causal networks from biological systems but without information on the timing of events.
“Our previous causal discovery tool can learn contemporaneous causal networks for a broad range of biological or biomedical data, from single cell gene expression data to patients’ medical records,” explains co-lead author Franck Simon, a research engineer at the Institut Curie, Université PSL, Sorbonne Université, France.
“However, live-cell time-lapse imaging data contain information about cellular dynamics, which can facilitate the discovery of novel cause-and-effect processes, based on the assumption that future events cannot cause past ones.”
Simon served as co-lead author of the paper alongside Maria Colomba Comes—at the time Ph.D. student in the Department of Electronic Engineering, University of Rome Tor Vergata, Rome, Italy, now researcher at the Tumours Institute of Bari, Italy—and Tiziana Tocci—a Ph.D. candidate at the Institut Curie, Université PSL, Sorbonne Université.
To explore this, CausalXtract reconstructs time-unfolded causal networks, where each variable is represented by several nodes at different timepoints. This factors in the links between successive time steps in the data. This graph-based causality goes beyond the early models of temporal causality (“Granger causality”) which can overlook actual causal effects, as demonstrated in the study.
“We benchmarked the tool using artificial datasets that resemble real-world data in terms of number of time steps and network size and found that it matches or out-performs existing methods while running orders of magnitudes faster,” adds Simon.
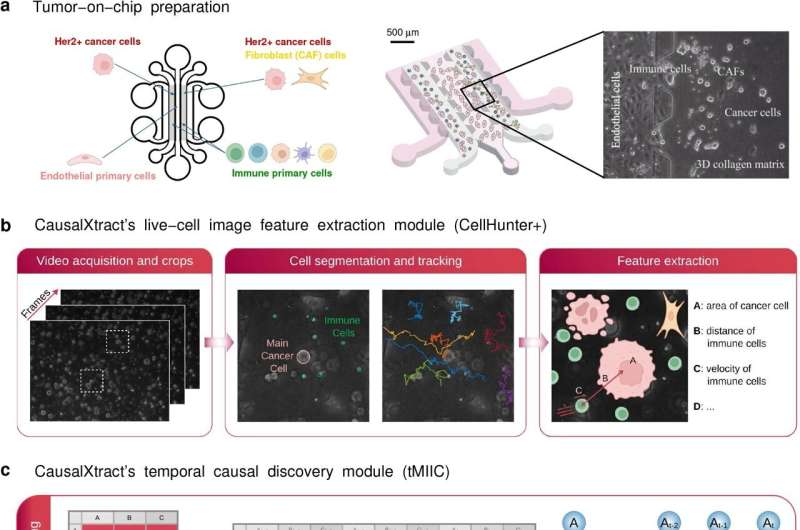
To test the tool’s performance with real biological data, the team used time-lapse image data from a tumor-on-a-chip model demonstrating the effects of the cancer drug, trastuzumab. A tumor-on-a-chip model replicates the 3D structure and microenvironment of a tumor, consisting of tumor cells, immune cells, tumor-associated fibroblasts and endothelial cells.
From this model, “we extracted cell features such as geometry, velocity, cell division, cell death, and transient and persistent cell–cell interactions from the raw images,” says Tocci. The team then reconstructed a time-unfolded causal network from information about the cell features, interactions and therapeutic conditions at different time points.
“This reconstruction revealed new biologically relevant insights and confirmed existing known relationships between cells,” says Maria Carla Parrini, co-author of the study, who oversaw the tumor-on-chip experiments at Institut Curie.
For example, the model confirmed that treatment with trastuzumab increases cell death and the number of interactions between cancer and immune cells, but it also showed for the first time that cancer-associated fibroblasts (CAFs) independently block cancer cell death. While it was already reported that CAFs reduce the effectiveness of treatment, these findings provide new insights into how this occurs.
The team was also interested to note that CausalXtract identifies opposing effects at different timepoints. For example, it captured that a cell’s eccentricity—how much the cell deviates from its normal circular shape—changes at different phases of cell division.
Late phases of cell division are linked with an increase in cell eccentricity, but this is preceded by a decrease in eccentricity 2–4 hours before the cell splits, once the decision to divide has been made. This demonstrates the tool’s potential for uncovering novel and possibly time-lagged causal relationships between cellular features.
“CausalXtract opens up new avenues to analyze live-cell imaging data for a range of fundamental and translational research applications, such as the use of tumor-on-chips to screen immunotherapy responses on patient-derived tumor samples,” says co-senior author Eugenio Martinelli, Full Professor at the Department of Electronic Engineering, University of Rome Tor Vergata.
“With the advent of virtually unlimited live-cell image data, flexible interpretation methods are much needed, and we believe that CausalXtract can bring unique insights based on causal discovery to interpret such information-rich data,” adds co-senior author Hervé Isambert, Group Leader DR CNRS, Institut Curie, Université PSL, Sorbonne Université.
More information:
Franck Simon et al, CausalXtract: a flexible pipeline to extract causal effects from live-cell time-lapse imaging data, eLife (2024). DOI: 10.7554/eLife.95485.1
Journal information:
eLife
Citation:
Computational tool can pinpoint causal relationships from complex biological data (2024, September 17)
retrieved 17 September 2024
from https://phys.org/news/2024-09-tool-causal-relationships-complex-biological.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.