A new mucosal COVID-19 vaccine poised to revolutionize the delivery process is especially beneficial for those with a fear of needles.
A next-generation COVID-19 mucosal vaccine is set to be a game-changer not only when delivering the vaccine itself, but also for people who are needle-phobic.
New Griffith University research, published in Nature Communications, has been testing the efficacy of delivering a COVID-19 vaccine via the nasal passages.
Professor Suresh Mahalingam from Griffith’s Institute for Biomedicine and Glycomics has been working on this research for the past four years.
Benefits of Live-Attenuated Vaccines
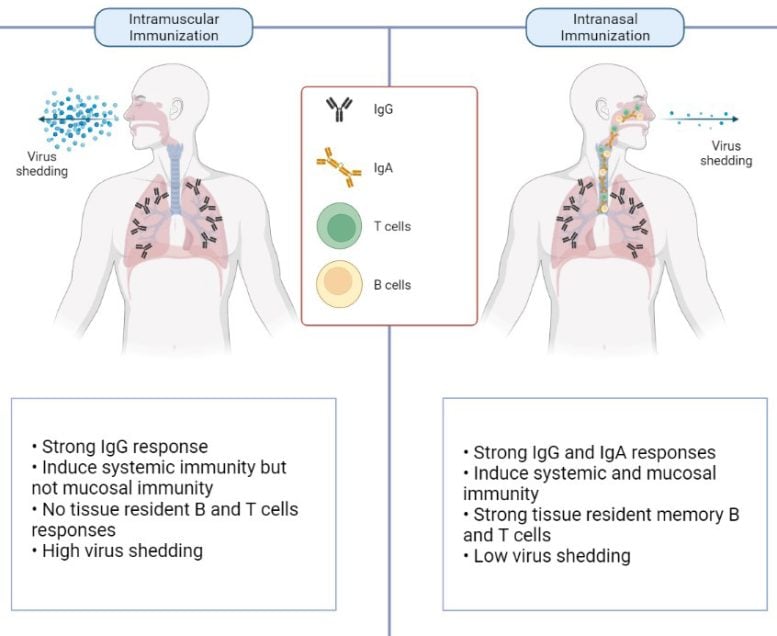
“This is a live attenuated intranasal vaccine, called CDO-7N-1, designed to be administered intranasally, thereby inducing potential mucosal immunity as well as systemic immunity with just a single dose,” Professor Mahalingam said.
“The vaccine induces strong memory responses in the nasal mucosa offering long-term protection for up to a year or more. It’s been designed to be administered as a single dose, ideally as a booster vaccine, as a safe alternative to needles with no adverse reactions in the short or long term.”
Live-attenuated vaccines offer several significant advantages over other vaccine approaches.
They induce potent and long-lived humoral and cellular immunity, often with just a single dose.
Live-attenuated vaccines comprise the entire virus thereby providing broad immunity, in contrast to a single antigen which is used in many other vaccine platforms.
Lead author Dr Xiang Liu said the vaccine provides cross-protection against all variants of concern, and has neutralising capacity against SARS-CoV-1.
“The vaccine offers potent protection against transmission, prevents reinfection and the spread of the virus, while also reducing the generation of new variants,” Dr Liu said.
“Unlike the mRNA vaccine which targets only the spike protein, CDO-7N-1 induces immunity to all major SARS-CoV-2 proteins and is highly effective against all major variants to date.
“Importantly, the vaccine remains stable at 4°C for seven months, making it ideal for low- and middle-income countries.”
Licensing and Future Prospects
The vaccine has been licensed to Indian Immunologicals Ltd, a major vaccine manufacturer.
Dr. K. Anand Kumar, co-author of the publication and Managing Director of Indian Immunologicals Ltd. Said: “We are a leading ‘One Health’ company that has developed and launched several vaccines for human and animal use in India and are currently exporting to 62 countries.”
“We have completed all the necessary studies of this novel COVID-19 vaccine which offers tremendous advantages over other vaccines. We now look forward to taking the vaccine candidate to clinical trials.”
Professor Lee Smith, Acting Director of the Institute for Biomedicine and Glycomics, said he was delighted with the research findings.
“These results towards developing a next-generation COVID-19 vaccine are truly exciting,” Professor Smith said. “Our researchers are dedicated to providing innovative and, crucially, more accessible solutions to combat this high-impact disease.”
Reference: “A single-dose intranasal live-attenuated codon deoptimized vaccine provides broad protection against SARS-CoV-2 and its variants” by Xiang Liu, Wern Hann Ng, Eva Zusinaite, Joseph Freitas, Adam Taylor, Venugopal Yerragunta, Shukra Madhaha Aavula, Sambaiah Gorriparthi, Santhakumar Ponsekaran, Rama Lakshmi Bonda, Priyanka Mani, Sridevi V. Nimmagadda, Sainan Wang, Laura Sandra Lello, Ali Zaid, Ujjwal Dua, Sharon A. Taft-Benz, Elizabeth Anderson, Victoria K. Baxter, Sanjay Sarkar, Zheng L. Ling, Thomas M. Ashhurst, Samuel M. S. Cheng, Priyabrata Pattnaik, Anand Kumar Kanakasapapathy, Ralph S. Baric, Felicity J. Burt, Malik Peiris, Mark T. Heise, Nicholas J. C. King, Andres Merits, Rajendra Lingala and Suresh Mahalingam, 26 August 2024, Nature Communications.
DOI: 10.1038/s41467-024-51535-y