
Unlock the Editor’s Digest for free
Roula Khalaf, Editor of the FT, selects her favourite stories in this weekly newsletter.
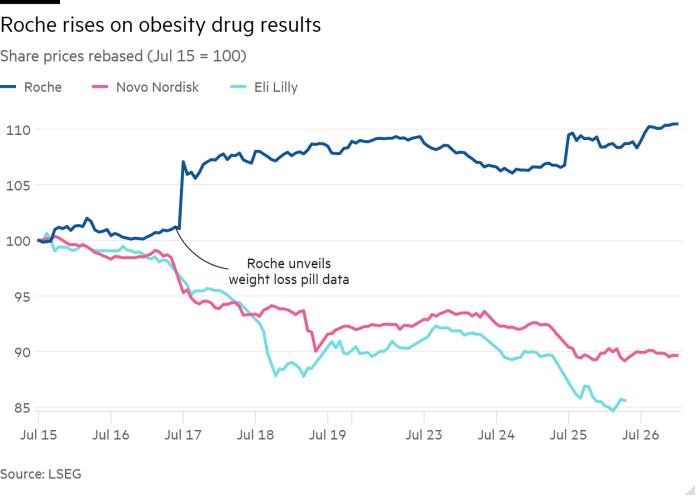
Roche plans to fast-track its anti-obesity drugs to challenge rivals Eli Lilly and Novo Nordisk in the booming market after unveiling promising data for a weight-loss pill.
Thomas Schinecker, chief executive of the Swiss pharmaceutical company, told the Financial Times that Roche’s first obesity drugs would come to market “significantly faster than people are expecting”, potentially by 2028.
The treatments, acquired in an up to $3.1bn takeover of biotech Carmot last year, includes a weight-loss jab set to enter phase II trials and a pill that has given users 6.1 per cent reduction in weight compared with a placebo after four weeks.
Schinecker said the company could have “around seven” drugs from the Carmot acquisition, with several in an earlier stage of development, although it has disclosed details for just three of the assets.
Data from its pill last week sent shares in the Swiss company up 6 per cent on the day of the release. The results also hit shares of Novo Nordisk and Eli Lilly over concerns that Roche could challenge their dominance in the sector.
Goldman Sachs analysts expect the obesity market to surpass $130bn by 2030, with several companies seeking to launch their own drugs.
But Roche will have to catch up with the two incumbents in the field, both of which are developing stronger and more effective treatments than their Wegovy (Novo Nordisk) and Mounjaro (Eli Lilly) drugs that have led to about 15 per cent and 20 per cent weight loss respectively in trials lasting more than a year.
Other drugmakers, including Boehringer Ingelheim and Pfizer, are also hoping to launch drugs, while shares in US biotech Viking Therapeutics rose 30 per cent on Thursday after it said it would advance an obesity pill to late-stage trials.
Schinecker said the company’s weight-loss pill will be more easily scalable as it is made synthetically, rather than through living natural molecules known as peptides, which is the case with the weight-loss pill being developed by Novo Nordisk.
Emily Field, an analyst at Barclays, said it was too early to tell if the company would be able to “disrupt the head start Novo and Lilly have”.
“If you look at what’s disclosed, it’s better than pretty much everything at four weeks. But there’s a lot we don’t know,” she said, pointing to a lack of detail on side effects from the drug, such as nausea and vomiting.
Schinecker took over as chief executive in March 2023 and, in the past year, Roche has cut 25 per cent of underperforming drugs in development. The group plans to focus on a smaller but more promising pool of drugs, including in obesity and Alzheimer’s.
“That creates room for new starts and things that we can bring in from the outside so that you don’t carry projects for too long and then you find resources that you could put to much more effective use to develop new medicines,” Schinecker told the Financial Times.
But its plans come after a series of research failures in recent years, including a late-stage trial for lung cancer drug tiragolumab this month. The treatment failed to increase survival rates compared with a Merck drug, Keytruda.
Schinecker said he hoped that Roche’s obesity drugs could also be used in combination with the company’s other treatments for conditions linked to obesity.
He pointed to the company’s blockbuster eye treatment Vabysmo, developed with its subsidiary Genentech, which made SFr1.8bn ($2.1bn) in sales in the first half of the year and has shown promise in treating diabetic macular edema.
Driven by strong sales of Vabysmo and multiple sclerosis drug Ocrevus, on Thursday the company raised its expected earnings per share growth in 2024 to the high single-digit range, up from previous guidance of a mid-single digit increase.