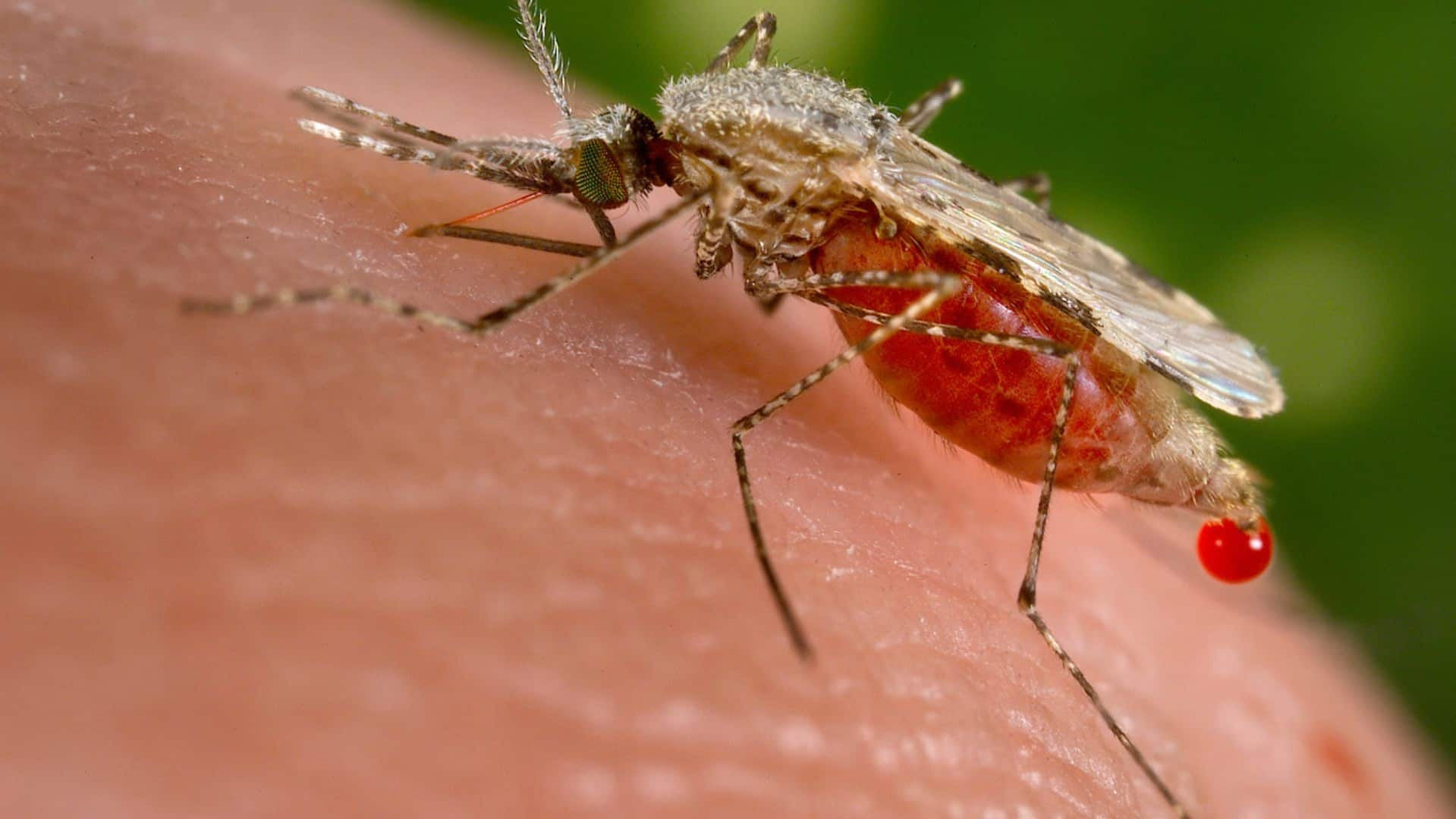
The World Health Organization authorized a second malaria vaccine on Monday, in a decision that could offer countries a cheaper and more readily available option than the world’s first shot against the parasitic disease.
WHO director general Tedros Adhanom Ghebreyesus said the UN health agency was approving the new malaria vaccine and recommending its use in children at risk of the disease, based on the advice of two expert groups — the Strategic Advisory Group of Experts on Immunization and the Malaria Policy Advisory Group.
“As a malaria researcher, I used to dream of the day we would have a safe and effective vaccine against malaria,” Ghebreyesus said. “Now we have two.”
Oxford University developed the new three-dose vaccine with help from the Serum Institute of India, which will mass manufacture it.
The Current19:28Vaccine could be a game-changer in the fight against malaria
Ghana is the first country in the world to approve the R21/Matrix-M from Oxford University, which could be a game-changer in the fight against malaria. Matt Galloway speaks with Adrian Hill, who led the design and clinical development of the vaccine; and Dr. Fred Aboagye-Antwi, a medical entomologist and parasitologist who works with the NGO Target Malaria.
Research suggests the vaccine, called R21/Matrix-M, is more than 75 per cent effective and that protection is maintained for at least another year with a booster. Tedros said the shot would cost about $2 to $4 US and could be available in some countries next year if funders agree to buy it.
Earlier this year, regulatory authorities in Ghana and Burkina Faso approved the vaccine.
Tale of 2 vaccines
“This is one more tool we will now have, but it’s not going to replace bed nets and spraying insecticides,” said John Johnson with Doctors Without Borders. “This is not the vaccine that’s going to stop malaria.”
Johnson was not part of the WHO expert group that gave the R21/Matrix-M vaccine the green light.
In 2021, WHO endorsed the first malaria vaccine in what it described as a “historic” effort to end the devastating toll the mosquito-transmitted disease has in African countries, which are home to most of the world’s estimated 200 million cases and 400,000 deaths.
But that vaccine, known as Mosquirix and made by GSK, is only about 30 per cent effective, requires four doses and protection fades within months. However, WHO experts said data to date on Mosquirix and R21 vaccines does not show which one is more effective.
The Bill & Melinda Gates Foundation, one of the GSK vaccine’s biggest backers, stepped back last year from financially supporting Mosquirix’s rollout, saying it was less effective than officials would like and that funding would be better used elsewhere. Demand for this vaccine “far exceeds supply” according to Ghebreyesus, who says the second vaccine will help fill the gap.
“The big difference with these two vaccines is access,” Johnson said, noting that only about a dozen countries are scheduled to get limited quantities of the GSK vaccine in the next few years.
GSK has said it can only produce about 15 million doses a year. The Serum Institute of India has said it could make up to 200 million doses of the R21 vaccine a year.
Not the ‘end of the malaria story’
Alister Craig, an emeritus professor at the Liverpool School of Tropical Medicine, said he would recommend countries trying to get the Mosquirix vaccine switch to the R21 vaccine instead.
If the new vaccine is rolled out widely across Africa, it could dramatically reduce the amount of severe illness and deaths caused by malaria in a few years, Craig said.
Neither of the malaria vaccines stop transmission so immunization campaigns alone won’t be enough to stop epidemics. Efforts to curb the disease are also being complicated by increasing reports of resistance to the main drugs used to treat malaria and the spread of invasive mosquito species.
“You would be foolish to think that this vaccine is going to be the end of the malaria story,” Craig said.
Still, it’s a tool that will “help bolster malaria prevention and control efforts,” according to Dr Matshidiso Moeti, WHO regional director for Africa. Disruptions in malaria-related health services between 2019 and 2021 due to the COVID-19 pandemic led to an additional 63,000 deaths, according to the organization.
“WHO is now reviewing the vaccine for prequalification, which is WHO stamp of approval, and will enable GAVI (a global vaccine alliance) and UNICEF to buy the vaccine from manufacturers,” Ghebreyesus said.
The U.S. has reported ‘domestically acquired’ cases of malaria for the first time in 20 years, and experts say the disease could become more common because of climate change.
Dengue vaccine gets approval
In a separate decision, WHO’s expert group also authorized the dengue vaccine made by Takeda, a Japanese pharmaceutical company, which was previously approved by the European Union drug regulator.
There is no specific treatment for dengue, common in tropical Latin American and Asian countries. While most infections are mild, severe cases of the mosquito-spread disease can lead to internal bleeding, organ damage and death.
Nearly 1,000 people have been killed by dengue this year in an ongoing epidemic in Bangladesh, the country’s worst outbreak of the disease.
Previous studies have shown Takeda’s vaccine was about 84 per cent effective in preventing people from being hospitalized with dengue and about 61 per cent effective in stopping symptoms four years after getting immunized.
WHO’s expert groups advised that the Takeda dengue vaccine be used in children aged 6 to 16 in countries with a high prevalence of the disease.